- ≡ Tortula australasiae Hook. & Grev., Edinburgh J. Sci. 1: 301 (1824)
- ≡ Barbula australasiae (Hook. & Grev.) Brid., Bryol. Univ. 1, 828 (1827)
- ≡ Didymodon australasiae (Hook. & Grev.) R.H.Zander, Phytologia 41: 21 (1978) – as Didymodon australasii
- = Trichostomum gracile R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 486 (1897) nom. illeg., non Trichostomum gracile Hornsch. ex Fürnr. 1827
- = Weissia acutifolia R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 438 (1899) nom. illeg., non Weissia acutifolia (H.Philib.) Kindb. 1898
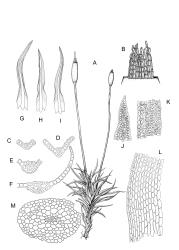
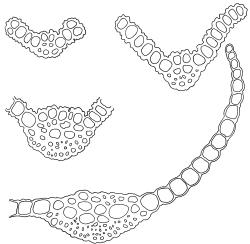
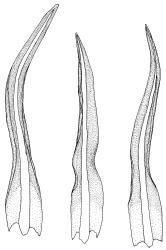
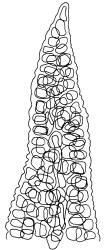
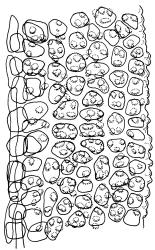
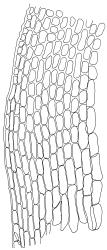
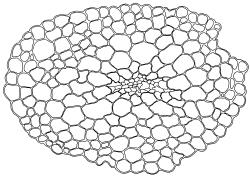
Plants blackish green, white below, tufted or in turves. Stems 3–10 mm, branched from below perichaetia and perigonia, in cross-section with a central strand, sclerodermis absent. Leaves erect-spreading from a loosely sheathing base with distal part often ± erect when moist, loosely curled individually and often around the stem when dry, 1.5–2.5 mm, narrowly linear-lanceolate, carinate, acute to acuminate; margins variably recurved, becoming bistratose distally, papillose; upper laminal cells somewhat obscure, irregularly quadrate, rounded or oblate, firm-walled, superficially ± flat, with 2–4 low, simple, often inconspicuous papillae, 8–13 × 9–12 µm, becoming longer towards the leaf base; lower laminal cells conspicuously differentiated, lax and thin-walled, hyaline, smooth, rectangular, becoming narrower and firmer towards the leaf margins. Costa reddish brown, stout below, narrower above and sunken in a groove, failing before the apex or percurrent; adaxial superficial cells similar to adjacent laminal cells except in extreme leaf base; abaxial superficial cells quadrate distally. Brood bodies not seen. Axillary hairs of 4–8 cells, of which the basal cell is shorter and brown. Laminal KOH colour reaction negative, or pale yellow, sometimes with red patches.
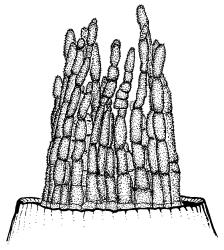
Dioicous. Perichaetia terminal, with perichaetial leaves erect, loosely sheathing the seta base, longer than vegetative leaves, with basal zone of smooth, hyaline, lax cells extending >½ leaf length. Perigonia terminal, often overtopped by an innovation, bulbiform, on equal-sized plant. Setae slender, reddish orange, 4–11 mm. Capsules erect, symmetric, cylindric or narrowly ellipsoid, brown to reddish brown, darker at the mouth, 1–1.5 mm. Operculum rostrate with a slightly curved oblique beak, from ½ to equal the theca length, with cells arranged obliquely in the base only. Peristome orange-brown, c. 200–400 µm (as retained on theca after capsule dehiscence, but with additional fragments remaining within the operculum), comprising 16 ± erect teeth (incurved when dry), inserted on a low basal cylinder and divided, sometimes irregularly, into 2 finely and densely spiculose rami. Calyptra cucullate. Spores 1–17 µm, smooth.
Sainsbury 1955, plate 29, fig. 1 (as Barbula australasiae); Catcheside 1980, fig. 85; Beever et al. 1992, fig. 32; Jiménez 2006, fig. 6, G – H (as Didymodon australasiae); Lüth 2019, p. 611 (as Didymodon australasiae); Malcolm et al. 2020, pp. 514–517.
Trichostomopsis australasiae is distinctive in its slender habit, blackish green colour (but white below), and dry leaves often curled loosely around the stem when viewed from above. The statement by Scott & Stone (1976, p. 210), regarding southern Australian material (as Barbula australasiae), that dry leaves are "not wound round the stem" does not apply to N.Z. material. The narrowly linear-lanceolate leaves, often with a sigmoid stance when moist, are characteristic. Microscopically, the patch of hyaline, thin-walled cells in the leaf base, and the bistratose lamina margins near the apex are useful characters. The thickened margins are raised adaxially, so that the costa lies in a narrow groove in the acuminate leaf apex, giving a distinctive appearance to leaf tips under the microscope.
Trichostomopsis australasiae has been confused with species of Bryoerythrophyllum, which also have a zone of lax, thin-walled hyaline cells adjacent to the costa in the leaf base, but the unistratose leaf margins (vs bistratose in T. australasiae), and red tinge to the lower leaves of Bryoerythrophyllum (vs no red coloration in T. australasiae) will separate them.
K; NI: N Auckland, including offshore islands (PK, HC, LT, RT), S Auckland, Hawke's Bay, Taranaki, Wellington; SI: Nelson, Marlborough, Canterbury, Otago; St; Ch; A.
Widespread in both hemispheres. Tasmania*, North America*. Recorded from mainland Australia by Streimann & Klazenga (2002), from South America, northern and southern Africa, and Europe by Zander et al. (2007).
A very common species, occurring on a wide range of soil and rock types (e.g., limestone, basalt, schist, and Waitemata sandstone). In addition, it grows on highly modified substrates (e.g., concrete, mortar, midden material, soil treated with herbicide, and painted wood). The species is found most commonly in dry, insolated sites, but also tolerates light shade. Most herbarium records are from anthropic habitats, such as roadsides, urban gardens, and cemeteries, but also from naturally disturbed sites, such as sea-cliffs. Commonly associated mosses include Barbula unguiculata, Bryum argenteum, B. dichotomum, Ceratodon purpureus, Gertrudiella torquata, and Tortula muralis. It is typically a lowland moss; most records are from below 500 m, but extend to c. 1000 m at Lindis Pass, Otago L.D. (K.W. Allison 8010, CHR 570243).
N.Z. material agrees well in all respects with the protologue, except that the peristome of the Western Australian holotype is illustrated as being long, with teeth spiralled approximately twice (Hooker & Greville 1824, pl. XII). Peristome development is perhaps variable in this species, but twisting has not been observed on more than one occasion (J.A. Jiménez, pers. comm., 12 July 2016). In N.Z. material the peristome is, after operculum dehiscence, seen to be short (c. 350 µm), rather irregular, and with teeth erect or slightly oblique (Plate 14, fig. B). This matches descriptions and illustrations of specimens from elsewhere in the southern hemisphere: southern Africa (Magill 1981) and southern Australia (Scott & Stone 1976). However, in both N.Z. (e.g., CHR 570244) and non-type Western Australian material (e.g., PERTH 07266219), discontinuous fragments of peristome can sometimes be observed through the wall of an in situ operculum extending nearly the full length of the rostrum. This suggests that potential for the formation of a long peristome is present in the genome and is variably expressed. No intact peristomes were available for study in the holotype (BM 000728645), but a single operculum still in situ has cells obliquely arranged in its conical base, but erect in the rostrum, as in N.Z. specimens. Thus the apparent discrepancies between the spiralled peristome illustrated in the protologue, and the shorter, straight or oblique peristome seen in other material, would not seem to justify a taxonomic distinction.
Material labelled "Tr. gracillimum" was interpreted by Dixon (1923 p. 114) as being "the plant published as Tr[ichostomum] gracile" by Robert Brown, and identified by Dixon as "Weisia weymouthii". A duplicate of the Brown collection (CHR 335725) is Trichostomopsis australasiae.
Robinson (1970) tentatively placed Trichostomum mokonuiense R.Br.bis (as T. makanuiense) in the synonymy of Trichostomopsis australasiae, perhaps following Dixon (1923 p. 128), who made an equivalent tentative synonymy in Barbula. Neither had apparently seen type material, for which details were given by Brown (1903). No type specimen has been located in either BM (L. Ellis, pers. comm., 31 Nov. 2016) or in Robert Brown’s herbarium in CHR. The habitat given by Brown in the protologue "on damp mud in the bed of the River", is highly unlikely for Trichostomopsis australasiae, and the description and illustrations given in the protologue are equivocal. The taxon is not considered further.