- ≡ Hookeria flexicollis Mitt. in Hooker, Handb. New Zealand Fl. 496 (1867)
- ≡ Eriopus flexicollis (Mitt.) A.Jaeger, Ber. Thätigk. St. Gallischen Naturwiss. Ges. 1875–1876: 338 (1877)
- = Eriopus brassii E.B.Bartram, Farlowia 4: 246 (1952)
- ≡ Calyptrochaeta brassii (E.B.Bartram) Streimann, J. Hattori Bot. Lab. 88: 108 (2000)
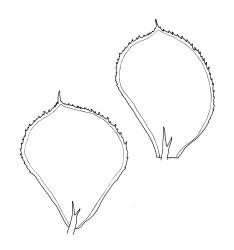
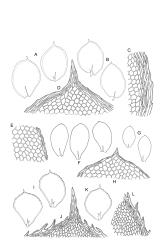
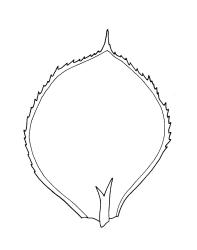
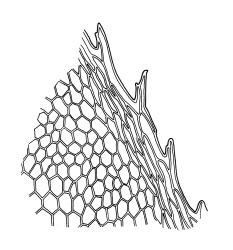
Plants medium-sized, rather rigid, yellow- to bright green, not or scarcely iridescent, forming loose turves. Stems erect, unbranched, red-brown, c. 5–25 mm long, in cross-section with a coloured, subcortical region of firm-walled cells, lacking a central strand, typically c. 300 µm diam. near base, beset with abundant, pale brown and smooth rhizoids at base and in leaf axils (often giving rise to chlorophyllose filaments with transverse walls in upper portion of stem). Shoots c. 3.0–4.0 mm wide. Leaves inserted in 6 or 8 ranks, uniformly spaced throughout the stem, moderately crisped when dry, those in dorsal and ventral ranks ± symmetric, broadly elliptic or obovate, those in lateral ranks asymmetric, broadly elliptic or obovate, tapered to strongly apiculate apices, plane at margins, bordered throughout, spinose-serrate or rarely merely denticulate in upper half, 1.5–2.5(–3.5) × 1.0–1.5 mm (often smaller in ventral and dorsal ranks and on lower stem); upper laminal cells firm-walled and moderately thickened in corners, 45–60 × 21–27 µm, becoming gradually longer and laxer towards base; marginal cells elongate, thick-walled, and porose, forming a concolourous border 3–6 cells and 30–45(–60) µm wide throughout. Costa very weak (sometimes absent), bifurcating at base or occasionally single, extending to c. ⅕ the leaf but generally shorter.
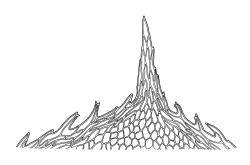
Dioicous. Perichaetia aggregated on ♀ stems; perichaetial leaves slenderly acuminate from an obovate base, ecostate, c. 2 mm. Perigonia ovoid, c. 1 mm, scattered on ♂ stems, with bracts ovate-obtuse and often retuse at apices, strongly concave, the inner typically yellow-brown, with 3–6 antheridia and lacking paraphyses. Setae 3.5–7 mm, straight below, arcuate below the capsule, stout (c. 180 µm diam.), yellow-brown, densely covered throughout with hyaline, non-papillose spines 45–90 µm long, with spines becoming abruptly longer (to c. 900 µm) and articulated by oblique walls to form a crest immediately below capsule; capsules inclined to horizontal, symmetric, 0.8–1.2 mm, smooth when dry, red-brown; operculum c. 0.5 mm. Exostome teeth c. 350 µm; endostome with cilia lacking. Calyptra c. 1 mm, densely hairy throughout or less often nearly smooth near apex, strongly fimbriate at base. Spores 15–21 µm, smooth or nearly so.
Streimann 2000, fig. 7, fig. 3 (as C. brassii, but this material not representative).
Calyptrochaeta flexicollis is most often confused with the smaller and more common Distichophyllum rotundifolium. The latter forms denser and more compact populations and has smaller leaves (0.7–1.3 vs 1.5–2.5 mm), which are denticulate rather than spinose-serrate. The costae in D. rotundifolium are mostly unbranched or spurred, and extend ⅔–¾ the leaf length in contrast to the bifurcate and much shorter costae of C. flexicollis; upper laminal cell dimensions and the nature of their setae also readily differentiate the two species.
Confusion occasionally occurs between C. flexicollis and its congener C. cristata, but the present species is a less robust plant, with shoots 3–4 mm wide vs 5–8 mm wide in C. cristata (other distinctions are noted under the latter species). Calyptrochaeta flexicollis is also confused with C. apiculata but the narrower leaf border and spinose-serrate margins of the former are usually sufficient to distinguish them. The setae of the non-coastal C. flexicollis are strongly spined while the setae of the salt-tolerant C. apiculata are smooth or papillose.
Calyptrochaeta flexicollis is distinguished from the South American C. setigera (Mitt.) W.R.Buck primarily by its stronger leaf border and its dioicous sexuality.
NI: N Auckland (Lake Rotokawau, Tūtāmoe Range), S Auckland (Moerangi), Gisborne (Manuoha, Te Wera Scenic Reserve, Whinray Scenic Reserve), Hawke’s Bay (Tarawera Homestead), Taranaki (Dawson Falls), Wellington (Erua, Akatarawa Range, Mt Munro Road, Wainuiomata–Orongorongo water catchment); SI: Nelson (near Collingwood, Reefton, Punakaikī area), Canterbury (Broad Stream, Banks Peninsula, Waimate), Westland (Camp Creek, Kellys Creek, Lake Paringa), Otago, Southland; St; A.
Austral. Tasmania*, mainland Australia*, Argentina*, Chile*.
A predominantly inland species growing on a variety of substrates in moist, shaded forests. It is widely distributed but rather infrequently collected in N.Z. It grows as an epiphyte (on Schefflera digitata, the podocarp Prumnopitys ferruginea, and the palm Rhopalostylis sapida), on rocks, soil, exposed roots, and logs in various stages of decay. Confirmed from c. 300 m (Akatarawa Range) to at least 900 m (Dawson Falls) on the North I., from sea level (Pororari River near Punakaikī and Taieri River mouth, Otago L.D.) to c. 900 m (Broad Stream) on the South and Stewart Is.
While C. flexicollis varies considerably in its stature, its overall aspect is quite distinctive; the combination of its usually modest stature, stout border, spinose-serrate margins, and strongly spinose setae make this beautiful species easily recognised.
Sainsbury (1956) recorded this species from Tasmania. I have confirmed several specimens from Tasmania (in HO) but reject Sainsbury’s suggestion that Eriopus tasmanicus Broth. is a taxonomic synonym of C. flexicollis (it is here considered synonymous with C. apiculata). Streimann (2000) recorded C. flexicollis from Tasmania and Victoria. The placement here of C. brassii in synonymy extends the Australian range northward to include high elevation sites (Mt Finnigan and Mt Bellenden Ker) in Queensland. The leaves chosen for illustration by Streimann (2000, fig. 3, as C. brassii) are exceptionally weakly toothed for the species and the Queensland material (including the type) exhibits greater leaf margin variability than is suggested by his illustrations.
This species was first recorded from South America (Aisén Province in Chile) by Cardot & Brotherus (1923); the Halle collection (in UPS) that was the basis of their report has been confirmed. Other Chilean and Argentinian collections have been confirmed, including isotype material (CHR 637792) of C. odontoloma (Dusén ex Matteri) Matteri.