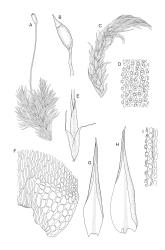
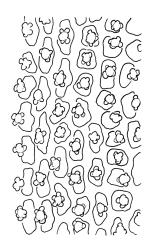
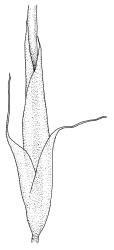
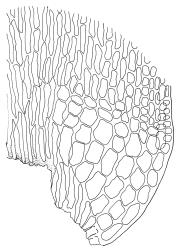
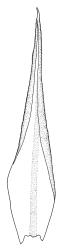
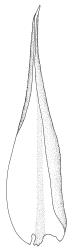
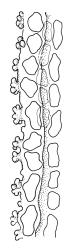
Plants medium-sized to robust, yellow- or grey-green, creeping, forming mats with dense masses of short branches. Stems prostrate, mostly <25 mm, wiry, giving rise to numerous short, erect branches, in cross-section of incrassate cells throughout, a poorly defined central strand apparently present, and the outermost 2–3 cell layers very incrassate and pigmented, with very sparse, thick-walled, smooth, and red-brown rhizoids. Branches fasciculate, self-supporting and erect, not or weakly curved when moist, curved away from the substrate or less often nearly straight when dry. Leaves of branches uniform in shape, narrowly ovate-lanceolate, slightly narrowed to insertion, bordered, uniformly tapered from widest point to apex, deeply concave below, becoming tubulose above, ± rugose and contorted when dry, entire or with a few weak teeth at extreme apex, concolorous throughout or with a short, white hair-point, 2.3–2.8 × 0.45–0.70 mm (under cover slip); mid laminal cells irregular in outline, mostly 8–15 × 7–8 µm and 1–2:1, strongly incrassate, with a single, large and much-branched papilla on the abaxial surface and smooth to moderately papillose on adaxial surface; toward leaf apex not differentiated (but the abaxial papillae often becoming longer and curved here), becoming elongate-rectangular, smooth, and porose at leaf base; cells of leaf border linear and smooth; the border usually (2–)3–4 cells wide at the widest part of leaf, tapering upwards and disappearing just below leaf apex; cells of the hair point elongate, lacking chlorophyll, often projecting at upper ends; cells at insertion strongly orange-pigmented in several rows, those near the costa linear-rectangular, smooth, and with strongly porose walls, those near the margin shorter and ± papillose; alar cells strongly differentiated, forming a large triangular group, orange or orange-brown, short-rectangular or subquadrate, with thin transverse walls and very thick and porose longitudinal walls. Costa lustrous in abaxial surface view, protruding abaxially, 0.1–0.125 the widest part of the leaf, disappearing below the leaf apex (obscure in upper part of leaf), in mid leaf cross-section with a central layer of guide cells and abaxial and adaxial stereid groups.
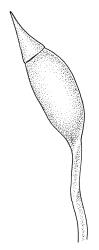
Apparently dioicous. Perichaetia lateral, the outer leaves little differentiated, the innermost shouldered, sheathing, hyaline for much of their length and with a strongly developed hair-point. Perigonia scattered on stems, conspicuous in moist material, the antheridia mixed with numerous brown filiform paraphyses. Setae one per perichaetium, elongate and straight, weakly sinistrorse, (7–)10–14 mm; capsules curved, nearly erect to strongly inclined, asymmetrically oblong-cylindric, 1.0–1.5(–1.8) mm, smooth both moist and dry, constricted below the mouth when dry (poorly illustrated here); exothecial cells subquadrate to ± irregular, firm-walled (transverse walls thinner than longitudinal walls); stomata not seen; annulus nil; operculum long-rostrate from a conic base. Peristome teeth inserted at mouth, red-brown, divided c. ½ to base into unequal segments, vertically papillose-striolate. Calyptra cucullate, smooth. Spores spherical, unicellular, green, mostly 25–39 µm.
Hooker 1819–1820, tab. 172 (as Leucodon pallidus); Brotherus 1924, fig. 167 f–h (as Dicnemoloma sieberianum); Crum 1986, figs 1–8; Malcolm & Malcolm 2003, p. 62; Meagher & Fuhrer 2003, p. 81.
Because of its overall habit, pale coloration, and bordered and rugose leaves with papillose cells, confusion is most likely with Mesotus celatus. When moist, plants of S. pallidum lack the sigmoidally spiralled leaves of M. celatus. Sclerodontium pallidum nearly always has some hair-pointed leaves, while these do not occur in Mesotus. The sporophytes of Sclerodontium are strongly exserted, while those of Mesotus are immersed. Under the microscope the multifid and single laminal papillae in S. pallidum contrast with the unbranched and multiple papillae of M. celatus. The spores in S. pallidum are unicellular, while those of M. celatus are multicellular. The northern distribution and mostly epilithic substrate of S. pallidum contrasts with the N.Z.-wide distribution and epiphytic substrate of M. celatus.
Confusion seems less likely with Racomitrium crispulum s.l. that occurs in similar dry epilithic habitats. Racomitrium crispulum s.l. is a darker plant with plane or reflexed leaf margins, a single well-developed plica in the lower leaf and non-papillose upper laminal cells with strongly sinuose walls.
NI: N Auckland (many localities) including offshore islands (HC, GB, LB, RT, Waiheke I.) S Auckland (Hauturu (Clark’s) I., Karangahake Gorge, Waikato River (site now submerged by Lake Karapiro), near Ātiamuri), Gisborne (Kawakawa Bay, Te Araroa); SI: Marlborough (without locality); Ch.
Australasian. Tasmania*, mainland Australia* (all mainland states, but apparently not NT). The only material seen from Tasmania is a specimen without locality or collector in the Mitten herbarium at NY. The species is accepted from Tasmania by Dalton et al. 1991 (as Dicnemoloma pallidum). Reported from Lord Howe I., New Caledonia, and Kerguelen by Crum (1986). The last locality seems phytogeographically highly unlikely and is based on an early (J.D. Hooker) collection in one herbarium.
Sclerodontium pallidum has a strongly northern distribution in N.Z. and is a widespread and common species in N Auckland L.D., becoming much rarer outside this L.D. It favours exposed boulders or outcrops of relatively base-rich but typically non-calcareous rocks (especially lava, basalt, and andesitic conglomerates). It is often in very dry sites that are sometimes in proximity to watercourses and perhaps rarely subject to flooding. A Chatham I. record (P.J. de Lange & P.B. Heenan CH983, AK 301023; CHR 592090) is unusual as it grew on limestone. It came from a dry, sunny site and grew in association with Pellaea rotundifolia and Camptochaete deflexa. Material has been seen from pōhutukawa (Metrosideros excelsa) roots among petrel burrows on Hauturu (Clark’s) I. near Whangamatā, S Auckland L.D. (P.J. de Lange 8305, CHR 587407), and there are a small number of records from pōhutukawa (Metrosideros excelsa) from Rangitoto and Great Barrier Is (collected by both J.E. Beever and J.K. Bartlett). Material recorded from a Cladium [Baumea] swamp without further information [K.W. Allison 403 (CHR 609473A, 609473B)] is, however, associated with Rosulabryum billardierei and Frullania falciloba, suggesting an epilithic origin. A collection from a mangrove lagoon on Rangitoto I. also was epilithic (L.B. Moore s.n., CHR 104954). The only South I. material seen is a J. McMahon collection from Marlborough L.D., without detailed locality data. In Australia S. pallidum grows most often on siliceous rock, especially sandstone.
On the North I. this species ranges from near sea level (Tī Point, North Auckland L.D.) to at least 400 m (Whangārei Heads, North Auckland L.D.). The above-mentioned corticolous collection from Mt Hobson is from 610 m elevation. Frequent associates include Breutelia pendula, Campylopus introflexus, Hypnum cupressiforme, and Rosulabryum billardierei as well as the lichens Cladia aggregata and Parmotrema reticulata.
Sclerodontium pallidum is an easily recognisable plant, which forms dense, pale, and creeping mats over rock. The upright branches are usually curved away from the substrate when dry, and the leaves are pale (due to their laminal papillae), tubulose, usually weakly hair-pointed, and finely rugose with dry. The lustrous and abaxially protruding costa contrasts sharply with the lamina, due to its smooth and elongate abaxial cells. The laminal cell papillae are large, flat-topped, and multifid at their apex. The abaxial laminal papillae are more robust than the adaxial and are best observed on the edge of a folded leaf under the microscope. In surface view the papillae appear stellate. The papillae near the leaf apex, particularly in leaves lacking a hair-point, are usually curved acropetally.
A second subspecies, S. pallidum subsp. celebesiae (Broth.) H.A.Crum, is restricted to Indonesia and, fide Crum (1986), is differentiated by "geographic separation, uniformly slender stature, and uniformly narrow leaf bases". The autonymic subspecies designation [Sclerodontium pallidum subspecies pallidum], dictated by the International Code of Nomenclature, is given at the top of this discussion, but the Australasian material of this species is so widespread, common, and distinctive that the repeated application of a subspecies name to N.Z. collections would seem pedantic.
Type material in the Hooker herbarium of Leucodon pallidus Hook. bears extensive notes in the script of Wilson: "I have reason to believe this to be the original of Leucodon pallidus Hook., Musc. Exot. T. 172. (Dicranum hypnoides Brown) of which no named authentic specimen exists in the Hookerian Herbarium. W. Wilson. April 4, 1845." The several plants in this specimen are unquestionably referable to Sclerodontium pallidum and no purpose would be served by questioning Wilson’s assessment. One of the mounted plants bears a strong resemblance to the habit drawing included in Musc. Exot. Tab. 172 (Hooker 1819–1820), and the annotation "Mr. Dickson" on the specimen corroborates the statement "a Dicksono et Hobsono receptus" in the protologue. The Hookerian herbarium mentioned by Wilson is presumably the W.J. Hooker herbarium of 1845, rather than the Herbarium Hookerianum incorporated into the Kew herbarium in 1867. Crum (1986) indicated that the type of Leucodon pallidus was collected at Port Jackson; the material at BM supporting this has not been seen.