- = Fabronia octoblepharis C.Knight, Trans. & Proc. New Zealand Inst. 8: 312 (1876) nom. illeg., non Fabronia octoblepharis Schwägr. 1816
- ≡ Fabronia antarctica Paris, Index Bryol. Suppl. 154 (1900) nom. nov. pro Fabronia octoblepharis C.Knight 1876 (non Fabronia octoblepharis Schwägr. 1816)
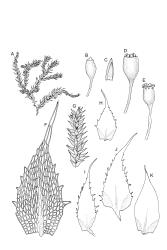
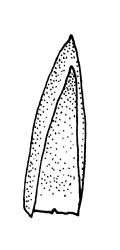
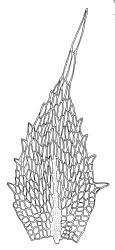
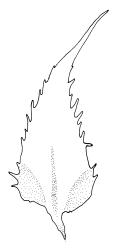
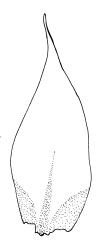
Plants bright green when fresh. Stems very fine, (5–)10–15 mm, in cross-section with a small central strand and 2 layers of firm-walled cortical cells. Branches variable in length, curved (especially when dry). Leaves secund when dry, ovate-lanceolate, gradually tapered to a slender and ± hyaline acumen or occasionally merely acute, 0.45–0.7 × 0.17–0.25 mm (to 0.9 × 0.45 mm in entire-leaved populations), variably spinose-toothed or entire, the teeth, if present, unicellular, to 45(–75) µm long, acute, and spreading at various angles in a single leaf; upper laminal cells rhombic-hexagonal, mostly 24–30(–45) × c. 9 µm (but longer in acumen); alar cells differentiated in a large ± triangular group, quadrate or oblate, extending to costa base and (7–)10–15 or more cells up the margin. Costa often indistinct, extending ⅓ –½ the leaf length.
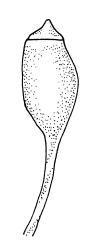
Autoicous. Perichaetia clasping the seta base, the inner leaves c. 0.8 mm. Perigonia scattered on stems, c. 0.4 mm, the perigonial leaves little differentiated from vegetative leaves in shape, apparently ecostate. Setae straight, 2.5–6 mm, twisted to the right; capsules obovoid-cylindric, 0.6–0.8 mm, pale brown at maturity; mouth equal to the capsule diameter; exothecial cells highly irregular and sinuose; operculum low-mammillate. Exostome teeth inserted at mouth, fused to form 8 broadly triangular pairs, irregular at apex, dark brown, striate on outer surface, coarsely papillose on inner surface, lacking trabeculae. Spores spherical to ellipsoid, bright green, (13–)15–21(–26) µm in greater diameter, rather thick-walled and coarsely low-insulate.
Knight 1876, p. 11; Scott & Stone 1976, pl. 84; Catcheside 1980, fig. 202.
When the leaf margins are toothed, F. australis could hardly be confused with anything else in the N.Z. flora. The teeth spread from the leaf axis at various angles with a few spreading at c. 90° (usually on the lower margins). The silky appearance, very small (well under 1 mm) leaves, weak costa and the characteristic large group of quadrate alar cells are highly distinctive, as are the nearly constantly present capsules. Ischyrodon lepturus is a larger plant with linear laminal cells; it is dioicous and not known to fruit in N.Z. Fabronia australis is primarily epiphytic and occurs mostly in inland sites, while I. lepturus is terrestrial and restricted to coastal sites. Confusion between entire-leaved forms and Brachythecium velutinum is possible, but the present species has generally smaller leaves, rhombic-hexagonal laminal cells, less-developed costae lacking terminal spines, and completely different capsules. The weakly developed costae, the larger alar group (often extending nearly to the costa), secund leaves, silky appearance, and a different habitat distinguish F. australis from Amblystegium serpens, q.v.
NI: K; N Auckland, S Auckland (Rotorua), Gisborne (Te Kaha, Poverty Bay), Hawke’s Bay (Wairoa, Wakarara Range, Pētane), Wellington (Masterton, Mt Bruce, Turakirae Head); SI: Nelson (Stoke, Nelson Botanical Garden, Upper Wairau Valley), Marlborough, Canterbury, Otago, Southland; St.
Australasian. Mainland Australia*. Recorded from Tasmania by both Scott & Stone (1976, p. 434) and Dalton et al. (1991).
On tree trunks, rock (including basalt and limestone), and rarely soil. The species is largely restricted to drier regions and on the South I. occurs mostly east of the Main Divide. Host species include a wide range of both indigenous trees (including Cordyline australis, Corynocarpus laevigatus, Griselinia littoralis, Metrosideros excelsa, M. kermadecensis, Myoporum laetum, Pennantia corymbosa, Pseudopanax arborea, Sophora spp., and Podocarpus totara) and adventive trees (including Populus and Salix spp., Sambucus nigra, Cupressus macrocarpa, and Pseudotsuga menziesii). Ranging from near sea level to c. 800 m.
Populations with entire or very nearly entire leaf margins predominate on North I. and tend to have larger spores (mostly 18–24 µm, rarely to 26 µm) and wider leaves (occasionally to 0.45 mm) than toothed populations. Entire-margined populations are exceedingly rare on South I. Populations with spinose-toothed leaf margins occur throughout the N.Z. range and plants in single populations (as at Turakirae Head, Wellington L.D., CHR 477491 and 477492) can have both entire and distinctly toothed leaf margins. Such variable populations suggest it would be an oversimplification to taxonomically recognise entire-leaved, large-spored populations in N.Z., even at the varietal level.
In the South American F. ciliaris, Buck (1983, p. 252) noted a weak correlation between entire margins and relatively large spore sizes; he chose to emphasise such variation by the recognition of varieties. Buck termed entire-margined material F. ciliaris var. polycarpa "the most common Fabronia in South America". He also suggested that South American species of Fabronia develop marginal teeth more strongly when growing in well-lit situations, while plants from shaded sites have weakly developed teeth or entire margins.
Isotype material of F. australis has leaf margins toothed with individual teeth short (9–18 µm). The marginal teeth of N.Z. populations are rarely greater than 45 µm from their acute basal angle to their apices. However, a small number of collections, including the non-localised holotype of F. octoblepharis and material from the Lyttelton Hills (Canterbury L.D., CHR 527737), have marginal teeth to c. 75 µm. Such material is interpreted here as merely an environmental variant unworthy of taxonomic recognition.
The suggestion that F. australis might be conspecific with the widespread northern hemisphere F. ciliaris (Brid.) Brid. (or F. octoblepharis Schwägr.) was first made by Dixon (1927, p. 278). More recently, Scott & Stone (1976, p. 434) suggested that Australasian material might be "only a facies" of F. ciliaris. However, the spores of N.Z. material of F. australis are larger than those recorded for F. ciliaris from either Europe (Limpricht 1890–1895) or North America (Crum & Anderson, 1981, p. 832) and it is therefore preferable to retain the name F. australis for Australian and N.Z. material. A monographic study could well produce a different conclusion.