- ≡ Funaria muhlenbergii Turner, Ann. Bot. [König & Sims] 2: 198 (1805)
- = Funaria glabra Taylor, London J. Bot. 5: 57 (1846)
- = Funaria tasmanica Müll.Hal. & Hampe, Linnaea 26: 490 (1855)
The following description is based on type material of Funaria glabra Taylor, and altered using N.Z. specimens.
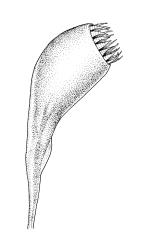
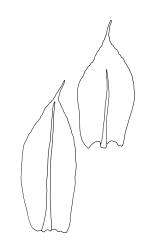
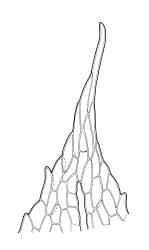
Plants yellow-green. Stems red-brown, c. 2–4 mm, usually branching once by subperigonial innovation, beset below with smooth, red-brown rhizoids. Leaves erect-spreading, oblong-obovate, 2.0–2.9 × 0.7–1.0 mm, concave, bluntly toothed in upper ⅓ by projecting cells, tapered in upper ⅓ to an acuminate apex; upper laminal cells oblong-hexagonal, (45–)60–90 × c. 30 µm, more oblong and to c. 120 µm long below; marginal cells mostly ± differentiated (but sometimes scarcely differentiated in N.Z. material); apical cell elongate, 125–180(–300) µm, often ± yellow; alar cells not or weakly differentiated. Costa c. 45–60 µm wide near base, failing below the base of the acumen. Axillary hairs present.
Autoicous. Perigonia and perichaetia as per genus. Setae c. 5–7 mm in N.Z. material, pale red-brown, weakly dextrorse and weakly hygroscopic; capsules weakly inclined, asymmetric, oblong-obovoid and curved, (2.3–)2.5–3.2 mm, constricted below the mouth when dry, with a well-defined, strongly wrinkled neck c. ½ the capsule length; mouth equal the capsule diameter, oblique; exothecial cells with indistinct lumina in surface view, c. 60 µm long, in cross-section with strongly cuneate walls; operculum plano-convex. Peristome double; exostome teeth sigmoid, c. 300–330 × 75–90 µm, acute, vertically striate nearly throughout, coarsely baculate near apices, with weak marginal appendiculae upper ½, with trabeculae well developed on inner surface, not fused at apices; endostome well developed, segments as wide as and c. ¾ the height of the exostome teeth, striate-baculate below, baculate above. Spores 29–32 µm, coarsely baculate-insulate, apparently lacking trilete scars.
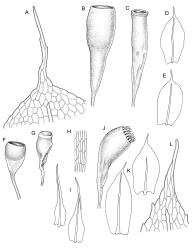
Smith 2004, fig. 164, 9–13.
NI: sine loc.; SI: Canterbury (Godley Head, Castle Hill Basin?), Otago (Arrow River, Mātukituki River).
Nearly cosmopolitan. Tasmania*, mainland Australia*, Africa* western North America*, Europe* including Great Britain*, Middle East*.
The collection from Godley Head was made from a silt bank associated with basalt bedrock at 120 m elevation; that from the Mātukituki River was collected from soil in a schist crevice at c. 300 m elevation. The Arrow River collection (D. Petrie, WELT M001297) bears no habitat details. There are two Colenso collections from an unknown North I. locality or localities; one was apparently segregated from a collection of Gigaspermum repens (suggesting an association with limestone).
The narrowly acuminate comal leaves with strongly elongate and yellow terminal cells, as well as the longer and more strongly defined capsule necks, distinguish this species from the much commoner E. radians. Poor specimens might be confused with the widespread E. apophysatus (where the comal leaf shape is very similar), but the present species has nearly entire leaf margins, asymmetric and peristomate capsules, and baculate-insulate and non-trilete spores. The probable record from the Castle Hill Basin is very scant and poor, but has perigonial leaves with narrowly acuminate apices, terminal cells c. 250 µm long, and a few capsules in the size range of the present species.
The South American Entosthodon laevis (Mitt.) Fife is closely allied (Fife 1987) and may eventually prove to be synonymous.