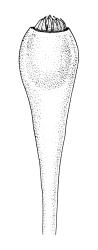
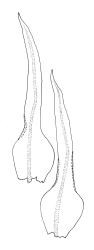
Plants yellow-green, becoming brown below, in tufts. Stems branched, to at least 15 mm. Leaves erect-spreading when moist, strongly contorted when dry, narrowly lanceolate from an ovate and pigmented base, acuminate, abruptly constricted and often narrowly reflexed above the base, entire, mostly 1.6–2.1 × 0.4–0.45 mm (under cover slip); upper laminal cells mostly oval or short-elliptic, some oblate, mostly 8–12 µm in greater diam., unistratose, mostly with a single mammilla over the lumen on both surfaces; basal interior cells linear-rhomboidal, smooth, yellow to orange, becoming porose towards leaf base, radiating weakly from the costa and gradually merging with cells of the upper lamina; cells of basal margins pale, forming a border c. 3–4 cells wide, extending nearly to the top of the base in most leaves. Costa ending shortly below the leaf apex. Gemmae absent.
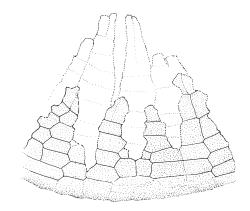
Gonioautoicous. Perichaetial leaves not differentiated or slightly less contorted than vegetative leaves when dry. Perigonia gemmiform, at base of perichaetium. Setae well-developed, c. 3–4 mm, weakly dextrorse when dry; capsules exserted, narrowly obovoid when moist, oblong-cylindric and 8-ribbed when dry, with a long, gradually tapered, and ± poorly defined neck, c. 2 mm; exothecial cells and stomata as per genus; operculum rostrate from a conic base, c. 0.5 mm. Peristome double; exostome teeth paired, broadly triangular, c. 300 μm, pale, with a median zig-zag line clearly visible on both surfaces, papillose-striolate, inserted very close to the capsule mouth, reflexed or ± erect when dry; endostome segments 16, c. ½ the height of the teeth, fragile (often not observable in N.Z. material), ± smooth, broad and irregular in outline, with a median zig-zag line of variable length; preperistome variably developed, sometimes rudimentary in N.Z. material. Calyptra campanulate, laciniate at base, densely hairy, enclosing nearly the entire developing capsule. Spores multicellular, mostly c. 6–8-celled, spherical to ovoid, mostly (65–)75–90(–120) μm in greater diam., obscurely papillose, sometimes germinating in capsule.
Malta 1933, figs 1c, 8, 9; Sainsbury 1955, pl. 35, fig. 2.
Ulota membranata can be difficult to distinguish from the more widespread U. viridis, particularly when the two species are growing mixed. The leaves of U. membranata are generally longer (1.6–2.1 vs 1.2–1.5 mm) and more strongly contorted when dry and the capsules decidedly more obovoid when moist. The large multicellular spores of the present species can usually be readily differentiated from the much smaller unicellular spores of U. viridis, even under the stereoscope.
Confusion is also possible with the more widespread U. lutea, another species with strongly contorted leaves when dry. However, the obovoid capsules of the U. membranata are usually readily distinguished from the more cylindric and more strongly ribbed capsules of U. lutea, and microscopic examination of the spores and endostome features will easily separate the two.
NI: Taranaki (Mt Taranaki), Wellington (several localities on Mt Ruapehu, Mauriceville, Ruahine Range); SI: Nelson (Iron Ridge, Mt Arthur Range, Anatoki River), Canterbury (Arthur’s Pass including Avalanche Peak), Southland (Mt Burns).
Australasian. Tasmania*.
Most records of this apparently uncommon species are from southern beech, probably Fuscospora solandri s.l. It occurs both on trunks and on small branches. One collection each from Dracophyllum sp. and Veronica glaucophylla has been seen. Ranging from c. 1125 to 1300 m on the North I. and from 850 to 1300 m on the South I. Apparently restricted to areas of high rainfall and often growing with U. viridis, Frullania spp., and Menegazzia nothofagi.
Ulota membranata is distinctive by its narrowly obovoid capsules when moist, its large, multicellular spores, and its endostome of 16 irregular and fragile segments. A preperistome may or may not be present. The peristome detail here (Image: Image\2FF2, D) does not illustrate the papillose-striolate ornamentation of the paired exostome teeth.
Although Malta (1933, p. 18) cited two N.Z. specimens in his protologue, he indicated (p. 19) that he considered the Tasmanian specimen (W.A. Weymouth 1652) to be the type. The HO isotype has multicellular spores mostly 75–81 µm, endostome segments nearly smooth, irregular in outline and mostly with a median line (but some lacking a median line and only one cell wide), and a rather conspicuous preperistome. Malta’s fig. 8, which illustrates a well-developed preperistome extending c. ⅓ the teeth height, is an accurate representation of the type collection.
In N.Z. material, preperistome development is variable, and in most collections it is either weakly developed or apparently lacking (e.g., A.J. Fife 7543 from Mt Arthur Range, CHR 438834; G.O.K. Sainsbury s.n., 10 Jan. 1945 from Mt Taranaki, WELT M021482). Tasmanian material (W.A. Weymouth 1653, packeted with type in HO) with a weakly developed preperistome has also been seen. All the N.Z. material examined has a stronger constriction above the leaf base than illustrated by Malta (1933, fig. 9). The margin near the constriction may be either narrowly reflexed or nearly plane.