- ≡ Splachnum purpurascens Hook.f. & Wilson, London J. Bot. 3: 539 (1844)
- ≡ Dissodon purpurascens (Hook.f. & Wilson) Müll.Hal., Syn. Musc. Frond. 2, 550 (1851)
- ≡ Eremodon purpurascens (Hook.f. & Wilson) Hook.f. & Wilson, Bot. Antarct. Voy. II (Fl. Nov.-Zel.) Part II, 94 (1854)
- = Splachnum purpurascens var. minor Hook.f. & Wilson, Bot. Antarct. Voy. I. (Fl. Antarct.) Part I, 123 (1845)
- = Dissodon purpureus Müll.Hal., Gen. Musc. Frond. 124 (1900)
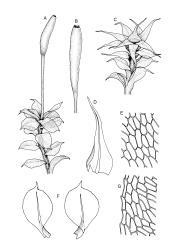
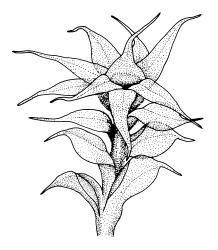
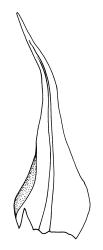
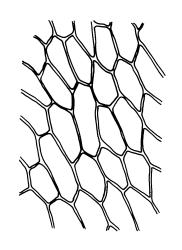
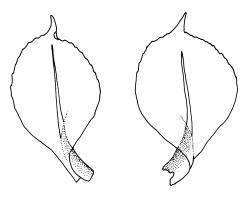
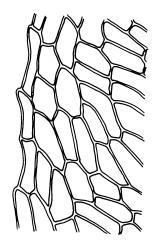
Plants variable in size, bright green to bright red-purple. Stems unbranched, typically 15–30 μm, beset with red-brown, smooth rhizoids. Leaves erect- to wide-spreading and nearly plane when moist, more erect and crisped when dry, becoming ± larger toward stem apex, broadly obovate, rather abruptly tapered to a reflexed or squarrose apiculus (0.5–0.9 mm long in well-developed leaves), plane or weakly recurved at margins, entire or weakly and obtusely toothed above, (2.3–)2.5–3.3 × (1.0–)1.5–2.0(–2.5) mm (exclusive of apiculus; leaves of male plants usually narrower than those of female plants); upper laminal cells thin-walled, weakly porose, oblong-hexagonal, in upper third c. (60–)75–105 μm, often becoming shorter towards the margins, arranged in ill-defined diagonal files, becoming longer and more oblong below. Costa rather ill-defined and stout, c. 100 μm wide at ⅓ above leaf base, dilated below, usually ending below the base of the apiculus. Axillary hairs inconspicuous.
Dioicous. Perichaetial leaves not differentiated. Perigonia terminal, ± globose, with ovate-lanceolate, widely-spreading bracts surrounding many antheridia intermixed with filiform, 6–7-celled paraphyses. Setae 10–15(–25) mm, straight, smooth, c. 300 μm diam., not twisted when dry, orange to red-purple; capsules erect, narrowly ellipsoid, with a tapering neck ⅓ –½ the total capsule length, c. 3.5–6 mm long, dark purple-brown or chestnut; exothecial cells oblate, very thick-walled, in ill-defined ranks; columella not or rarely protruding; stomata restricted to a narrow band at top of the neck; annulus weakly differentiated, apparently falling with the operculum; operculum rounded-conic. Peristome teeth inserted below the mouth, incurved or erect when dry, pale yellow- or red-brown, paired and longitudinally fused to form eight compound teeth, each pair broadly triangular and extending c. 175–200 μm beyond the mouth, c. 140 μm wide, finely and irregularly striolate on outer surface, sometimes ± vertically striolate above; preperistome not seen. Calyptra as per genus, 1.3–1.7 mm. Spores ± globose, 9–12 μm diam., smooth.
Wilson & Hooker 1845, tab. LVII, fig. v (as Splachnum purpurascens); Goffinet 2006, fig. 17 e–g.
Tayloria purpurascens varies markedly with respect to stature, pigmentation, and to a lesser degree, leaf form. Occasional forms occur with little or no secondary pigmentation, and leaf apiculi more strongly developed that usual, which could be confused with T. octoblepharum. The generally more obovate leaf form, non-excurrent costa, the reflexion of the apiculus, and the lack of salmon-pink pigmentation in the costa base are usually sufficient to permit its recognition, even in the absence of capsules. Perigonial bracts are considerably more ovate-lanceolate than the vegetative or perichaetial leaves, but they are erect-spreading, rather than rigidly erect as in T. callophylla.
There is a tendency for material from the subantarctic islands to have leaves which are less markedly obovate, and lurid rather than purple. Hooker 53 (from the Auckland Is), the type of "var. minor", and C. Meurk s.n., 13 Feb. 1971 (from Campbell I.) are examples of this expression, which is not deemed worthy of taxonomic recognition.
NI: S Auckland (Taupō), Gisborne (Lake Waikaremoana), Taranaki (single unlocalised collection), Wellington; SI: Nelson, Marlborough, Canterbury, Westland, Otago, Southland; St; Ch; Sn; A; Ant; C.
Probably endemic. Recorded from mainland Australia by Goffinet (2006) on the basis of a single poorly documented N.S.W. collection.
Occurring in a wide variety of vegetation types, including Leptospermum/Kunzea scrub, southern beech forest, broadleaved forest, and pākihi. It occurs on faeces of both herbivores (cattle, goats, etc.) and carnivores, and on decayed carcasses. A large fraction of collections are recorded from humus (on ledges, stumps, etc) and fail to mention the presence of dung; this is probably due to an advanced state of faecal decomposition. Often growing mixed with T. octoblepharum. It has an altitudinal range from near sea level (at least on South I. and subantarctic islands) to c. 1800 m (Travers Saddle, Nelson L.D.), but appears to be less frequent in the alpine zone than at lower elevations.
The only specimen seen from Taranaki is an unlocalised collection made by Miss J. Heywood in Feb. 1915 (BM). A possible Hawke's Bay specimen was collected by Sainsbury at Waimarino (BM). No material from Tasmania or mainland Australia has been seen.