Schlotheimia brownii sensu Sainsbury 1955, p. 238; non S. brownii Schwägr. 1826.
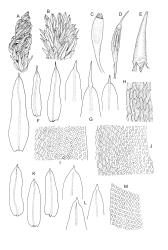
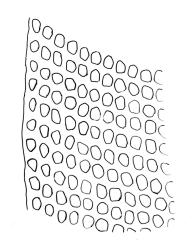
Plants small to robust, lustrous, forming dense cushions on bark or rock. Stems and rhizoids as per genus. Branches mostly branched by innovation, c. 3–20 mm. Branch leaves spiralled tightly or loosely around the branch when dry, erect-spreading when moist, oblong or ovate-oblong, broadly acute to obtuse, mucronate (costa mostly absent from mucro), keeled, indistinctly to moderately rugose (best seen when moist), often with a single plica of various length on each side of costa, mostly 1.5–2.5 mm, KOH negative or KOH positive chestnut-red; margins entire, narrowly recurved below, mostly plane above; upper laminal cells mostly oval to oblong, smooth, thick-walled, mostly 9–12 μm in greater diam., arranged in distinct oblique rows, juxtacostal cells mostly somewhat longer; marginal cells either not differentiated or slightly longer and oriented parallel to the margin but never forming a border; cells of the lower lamina mostly elongate-rhomboid, with very thick and ± curved walls, not or distinctly porose (often more distinctly porose adjacent to costa or at extreme base), usually strongly prorate at the upper abaxial surface of the cell but sometimes smooth or nearly so; cells at basal margins not differentiated. Costa mostly ending below the base of the mucro, occasionally short-excurrent into mucro, in lower leaf occupying less than 0.1 the leaf width and 30–45 μm wide, in cross-section as per genus.
Phyllodioicous. Perichaetia terminal, with perichaetial leaves scarcely differentiated or occasionally ± cuspidate. Male plants as per genus, frequent, c. 1 mm, the leaves acute, not mucronate. Setae c. 5–8 mm, slender, straight or slightly curved; capsules cylindric, exserted and erect, strongly 8-ribbed, c. 1.5–2.3 mm, with a short and weakly differentiated neck; exothecial cells thick-walled, mostly short-oblong, but some irregularly tapered at ends, mostly 30–60 μm, not in distinct vertical ranks; stomata relatively few, superficial, restricted to neck and the lowest ¼ or less of the urn (fewer than in S. campbelliana); annulus absent; operculum with a very long and fine rostrum from a conic base, c. ½ –⅔ the length of capsule. Peristome as per genus; exostome teeth 16, bright orange, c. 350 μm, ± reflexed when dry, with a distinct median line and coarsely transversely striate on outer surface; endostome of 16 (or 32?) segments, some irregular, from a very low membrane, coarsely striate throughout or ± baculate above, c. ⅔ the height of the exostome teeth. Calyptra with 4–6 tightly clasping, triangular basal lobes, c. 3 mm. Spores ± spherical, coarsely papillose, mostly 21–40 μm diam.
Although much N.Z. material of S. knightii was previously referred to S. brownii Schwägr. or S. brownii Brid. (by both Dixon 1926 and Sainsbury 1955), the Australian material of that taxon available for comparison has the leaves much more tightly spiralled around the branches when dry and is more strongly rugose than observed in S. knightii. The other differentiating features cited by Vitt (1989) in his key (particularly the dull leaf coloration, abrupt transition of cell size in the lamina) likewise seem to distinguish N.Z. material from S. brownii. Vitt’s (1989) decision to differentiate the N.Z. S. knightii from the predominantly mainland Australian S. brownii Schwägr. is followed here.
Schlotheimia knightii is most likely to be confused with Zygodon menziesii. Compared to that species, S. knightii is generally a more robust and mostly epiphytic plant with larger leaves, and a much more prominently red-brown tomentum. The lower laminal cells in S. knightii are elongate-rhomboid with very thick and ± curved walls, usually strongly prorate, and KOH negative or KOH positive chestnut. This contrasts with the predominantly epilithic Z. menziesii, in which the lower laminal cells are rectangular or oblong, ± thin-walled, smooth, and KOH positive yellow. If calyptrae are present, these readily differentiate the two species.
NI: N Auckland (Ōmahuta Forest, Waipoua, Titirangi), S Auckland (Ōtānepai Bush, Gisborne (Lake Waikaremoana), Hawke’s Bay (near Mōrere, Kaweka Range, Sunrise Hut, Makaroro (River?), northeast Ruahine Range), Wellington (Taihape, Erua Forest, Ōhakune, near Tangiwai, Ohau-iti River, Mt Holdsworth); SI: Nelson, Marlborough (Richmond Range), Canterbury, Westland, Otago, Southland; St. Vitt (1989, fig. 29) mapped the distribution and recorded a modest number of additional localities, particularly in the North I.
Endemic.
While primarily growing on the bark of living trees, S. knightii occasionally occurs on rock (sandstone, greywacke, and ultramafics). As with many predominantly epiphytic species, epilithic occurrences seem most frequent at higher elevations. Schlotheimia knightii is especially frequent on Fuscospora solandri s.l. It also occurs on Elaeocarpus dentatus, Fuscospora fusca, Griselinia littoralis, Knightia excelsa, Lophozonia menziesii, and Weinmannia spp., and the gymnosperms Dacrycarpus dacrydioides, Dacrydium cupressinum, and Prumnopitys taxifolium. Frequently associated bryophytes include Glyphothecium sciuroides, Holomitrium perichaetiale, Leptostomum inclinans, Lepyrodon australis, Macromitrium spp., Mesotus celatus, Ulota lutea, and Herbertus alpinus. On the North I. from c. 260 m (Waipoua, N Auckland L.D.) to at least 1200 m (Ōhakune, Wellington L.D.); on the South I. from near sea level (Papatōwai, Otago L.D.) to 1100 m (Waingaro River, Nelson L.D.).
It is surprising that there is no potential type material in any N.Z. herbarium, including WELT, which holds many of Knight’s original collections.
There appears to be some variation in response to 10% KOH solution in this species. The bulk of collections are KOH negative, but a number of herbarium collections have been seen in which the laminal cells exhibit a KOH positive chestnut-red reaction (e.g., Martin 405.14 from Marsden Road, Greymouth, Westland L.D., CHR 617331 and K.W. Allison 1651 from Waiau River, Southland L.D., CHR 611204). This variability does not seem to correlate with morphological differences.