- ≡ Barbula crinita Schultz, Nova Acta Phys.-Med. Acad. Caes. Leop.-Carol. Nat. Cur. 11: 226 (1823)
- = Trichostomum grossirete Broth. & Dixon in Dixon, J. Linn. Soc., Bot. 40: 444 (1912)
- ≡ Tortula flavinervis Dixon, Bull. New Zealand Inst. 3: 144 (1923) nom. nov. pro Trichostomum grossirete Broth. & Dixon 1912 (non Tortula grossiretis Cardot 1906)
- = Tortula flavinervis var. gigantea Dixon & Sainsbury, J. Bot. 71: 219 (1933)
Barbula pseudopilifera sensuSainsbury (1955)
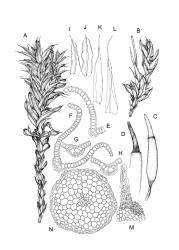
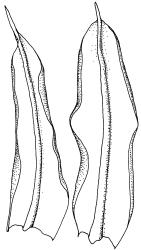
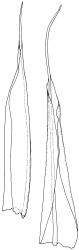
Plants yellow to yellow-green, forming dense turves on soil or rock. Stems 20–60 mm, simple or occasionally branched, sparsely tomentose below, in cross-section with central strand present, sclerodermis weakly developed. Leaves erect-spreading when moist, individually twisted and twisted around the stem with apices visible when dry, 2.5–4.5 mm, lingulate to lingulate-lanceolate, carinate, broadly acute to obtuse and often asymmetric at apex; margins ± narrowly recurved from base to shortly below apex, crenulate-papillose; upper laminal cells obscure, irregularly rounded-quadrate, densely pluripapillose with c. 6 complex papillae, (9–)12–16(–18) × (9–)11–14(–15) µm, becoming longer towards the base; upper marginal cells in one to several rows thicker-walled but not otherwise differentiated; lower laminal cells differentiated, colourless or sometimes yellow, long-rectangular or linear, thin- to firm-walled, smooth, and with several rows at the margin subquadrate to shortly rectangular, often chlorophyllose and forming a weak border. Costa excurrent as a long, concolorous, sometimes hyaline-tipped and weakly flexuose hair-point, which is smooth or slightly denticulate, up to ½ the length of the lamina, sunken in a deep groove; adaxial superficial cells variably short-rectangular to broad-fusiform and mostly pluripapillose with complex papillae; abaxial superficial cells long-fusiform, smooth to densely and finely papillose with simple papillae; in cross-section with 2 stereid bands. Axillary hairs of c. 10 cells, colourless throughout. Laminal KOH colour reaction yellow.
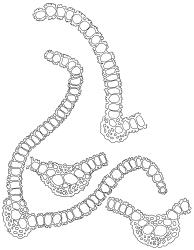
Dioicous. Perichaetia conspicuous with innermost leaves long-acuminate from an erect sheathing base, costa long-excurrent as a concolorous hair-point, margins plane, and laminal cells broadly fusiform and smooth. Perigonia bulbiform. Setae slender, flexuose, orange below and yellow above, 13–15 mm. Capsules long-cylindric and weakly arcuate, constricted below the mouth when dry, brown, glossy at the mouth, with a differentiated and persistent annulus, 2.0–3.0 mm. Operculum long conic and weakly curved, c. ¾ the theca length, with cells spirally arranged. Peristome of 16 pairs of pale filiform rami, each pair united by occasional anastomoses, densely spiculose, and several times twisted to the right, from a very short, perforate, basal cylinder. Spores 12–15 µm, very finely papillose.
Catcheside 1980, fig. 93 (as Barbula crinita); Magill 1981, fig. 69, 1–10 (as Barbula crinita); Zander 1993, pl. 26, figs 1–8; Malcolm et al. 2020, pp. 564–568.
Although Pseudocrossidium crinitum rarely produces capsules, it is readily identified on vegetative characters. The typically long excurrent costa alone will distinguish it from any N.Z. species of Barbula, Gertrudiella or Trichostomopsis. Barbula calycina is a similarly robust, yellow-green pottiaceous moss, but, in addition to the shorter excurrent costa, marginal laminal cells in the leaf base of B. calycina are similar to those nearer the costa (vs a marginal region of several rows subquadrate to shortly rectangular in P. crinitum).
The yellow-green coloration of the shoots of P. crinitum, with no hint of red, together with the long concolorous (not hyaline) hair-points, will usually distinguish this moss from species of Syntrichia. Many Syntrichia species show some red coloration, and may have excurrent costae that are at least partially red, or are colourless throughout. In cases of doubt, the yellow KOH reaction of leaf laminal cell walls of P. crinitum will readily distinguish it from any species of Syntrichia, the latter all giving a KOH red reaction. Confusion with Rosulabryum species, such as R. capillare, has occurred, but the smooth, hexagonal laminal cells of Rosulabryum readily separate them.
NI: N Auckland, S Auckland, Gisborne, Hawke’s Bay, Wellington (including KA); SI: Nelson, Marlborough, Canterbury, Otago, Southland; C.
Austral. Tasmania*, mainland Australia*, Southern Africa*, recorded from Chile by He (1998), and a depauperate form from Mexico and southwestern U.S.A. by Zander (1993)
Usually in well-insolated sites (including canopy gaps in forest), on soil, gravel, or rock, including limestone and basalt. The species grows with a wide range of other mosses characteristic of insolated sites, such as Fissidens megalotis, Hypnum cupressiforme, Thuidiopsis furfurosa, T. sparsa, and Triquetrella papillata.
Records range in elevation from near sea level to over 1000 m (Cobb Valley, Nelson L.D.).
The provenance of the type specimen of the basionym, given as "Java" in the protologue (Schultz 1823), is in doubt. Weber (1972) noted that neither Fleischer nor any other bryologist had subsequently found the species in Java, and "it becomes a distinct possibility that the type of the species could just as well come from an Australian source as from an African or South American one … and may never be known". The tortuous taxonomic and nomenclatural history of this moss is well summarised by Cano et al. (2016). Rather than declare Barbula crinita Schultz a nom. dub., we choose here to follow the nomenclature adopted by Zander (1993) and Cano et al. (2016, 2022). From a molecular and morphometric analysis of Pseudocrossidium crinitum specimens from South America, South Africa, and Australia, Cano et al. (2016) established that the taxon consisted of at least five different species (all previously described), which they considered could belong to different lineages in Pottiaceae. The four Australian specimens studied all segregated as P. crinitum s.s., and, although no specimens from N.Z. were included in the study, application of their Key to Species identifies the N.Z. moss as Pseudocrossidium crinitum s.s.