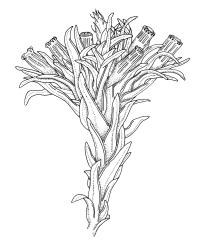
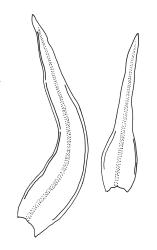
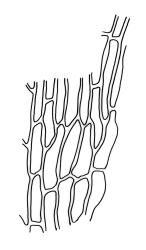
Plants 5–15 mm, tufted, bright green to olive-green above, yellow-brown to almost black below. Stems erect, much branched by innovation, with well-developed rhizoids near base. Leaves erect or slightly flexuose and sometimes twisted around stem when dry, spreading when moist, lanceolate to ovate-lanceolate, acute or rarely acuminate, entire or sometimes with protruding cell ends near base, recurved on both sides from just above the base to shortly below the apex, 2.2–3.1(–3.5) × 0.5–0.7 mm; upper laminal cells thick-walled, isodiametric or ± oblong, 9.5–16.0(–21.0) × 8.0–13.0 µm, papillose with 1–2 short and mostly unbranched papillae per cell; mid laminal cells more regularly oblong and usually more strongly papillose; basal interior cells rectangular to rhomboidal, thick-walled, nodose, and porose, weakly pigmented (but often yellow at extreme base), c. 21–60 × 8–10 µm, with several (to c. 10) rows at margins of oblong or rounded-quadrate cells and these clearly papillose to within c. 100–300 µm of the leaf base; papillae of marginal cells further up the margin tall (often 9–15 µm) and either branched or unbranched. Gemmae absent.
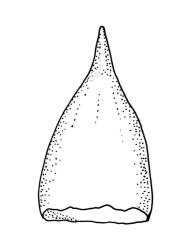
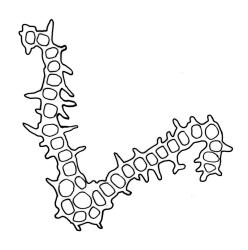
Gonioautoicous. Perigonia gemmiform, conspicuous below the perichaetia and on lower stem. Perichaetial leaves not differentiated. Capsules immersed to ± emergent, cylindric, strongly furrowed nearly to base and constricted below the mouth when dry (especially in older capsules), broadly ellipsoid and weakly or not furrowed when moist, 1 per perichaetium, often with several persistent on same shoot; exothecial cells oblong or rectangular, with ± thickened longitudinal walls, forming 16 alternate bands of relatively thin, unpigmented cells and relatively thick, more strongly pigmented cells (the latter c. 5 cells wide and forming ridges in dry capsules); stomata superficial, restricted to mid urn, very conspicuous. Peristome double; exostome teeth 16 or rarely paired to form 8, deep orange-red, recurved when dry, sometimes perforate along the middle line, the outer surface evenly and densely papillose, the inner surface with longitudinally oriented papillose ridges; preperistome absent; endostome well developed, with 16 variably pigmented (pale to bright orange-red) segments that are nearly as tall as the exostome, usually 1 cell wide except near base, with irregularly projecting margins, the outer surface smooth, the inner surface densely and finely papillose. Calyptra campanulate-mitrate, yellow or ± brown, and red-brown at apex, plicate, and ± split at base, naked or sparsely hairy (and then with hairs reaching or only slightly exceeding the top of calyptra). Spores globose, isosporous and 1-celled, 18–22.5 µm, papillose, often germinating within the capsule.
The majority of fruiting specimens of O. sainsburyi are easily recognised by the immersed to weakly emergent, strongly furrowed capsules with very prominent superficial stomata, 16 deep orange-red, revolute exostome teeth and 16 well-developed but narrow, orange-red, endostome segments. The very distinctive peristome often permits confident naming of well-developed plants under the stereoscope. However, many herbarium specimens, including many collected by Lewinsky, are sparse and difficult to confirm without causing unacceptable damage by dissection. There is some peristome variability in this species, with some material (e.g., J. Lewinsky 1679, between Twizel and Lake Pūkaki, Canterbury L.D., CHR 348742) having 8 paired and erect, rather than 16 recurved, exostome teeth.
When the capsules are poorly developed or old, O. sainsburyi can often be recognised by tall papillae in the marginal cells near the leaf base. These papillae extend downwards in at least some leaves to within c. 200 µm (sometimes less) of the leaf base; sometimes the cells must be viewed on edge to see the papillae. Progressing up the leaf margin the papillae rapidly increase in size and, at c. 300–600 µm above the leaf base, are typically up to 9–15 µm tall and often branched. By contrast, the papillae of the marginal cells c. 300–600 µm above the leaf base in O. hortense are shorter (<5 µm) and unbranched.
NI: Wellington (Kuratau River, near Lake Wairarapa); SI: Marlborough (Hāpuku River, Mt Fyffe), Canterbury (near Hawkswood, near Waikari, Cass, Geraldine, Lake Tekapō), Otago, Southland (near Gore). Lewinsky (1984) provides some additional records which have not been confirmed.
Endemic.
Mainly epiphytic and particularly common on willow. Lewinsky (1984, tab. 1) records it from three genera of native and six genera of introduced woody plants. It occurs rarely on non-calcareous rock (CHR 348718 from Mt Fyffe). Often occurring admixed with other Orthotrichum spp., Calyptopogon mnioides, and Syntrichia papillosa. Sparse material (which is often collected) with either immature or only older capsules can be difficult to name. Occurring from near sea level (Kaitangata, Otago L.D.) to at least 640 m (near Naseby, Otago L.D.). Orthotrichum sainsburyi avoids high rainfall areas on the South I. and remains known from very few localities on the North I.