- ≡ Schizymenium bryoides Harv., Gen. S. Afr. Pl. 385 (1838)
- = Mielichhoferia ecklonii Hornsch., Linnaea 15: 118 (1841)
- = Mielichhoferia australis Hampe, Linnaea 28: 204 (1856)
- = Mielichhoferia tenuiseta Mitt. in Hooker, Handb. New Zealand Fl. 750 (1867)
- = Mielichhoferia buchananii R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 443 (1899) – as buchanani
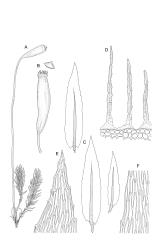
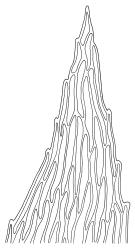
Plants pale green, lustrous, forming loose turves. Stems yellow- or dark-brown below, from < 5–c. 15 mm, usually several arising from a common point, otherwise unbranched except for subperichaetial innovation, sparsely beset below with yellow-brown and coarsely papillose rhizoids, in cross-section angled, with 1–2 layers of thick-walled and pigmented cortical cells and an ill-defined and small central strand. Leaves reduced on lower stem, larger and evenly distributed above, erect-spreading when moist, narrowly lanceolate, slightly narrowed at insertion, gradually tapered to an acute apex, mostly 0.8–1.3 × c. 0.25–0.4 mm (on upper stem), nearly flat, toothed nearly to base by blunt, wide-spreading and projecting cell ends, plane at margins; mid laminal cells linear-rhomboidal, mostly 90–150 × c. 10 µm, becoming ± shorter towards apex and insertion; marginal cells slightly narrower in a single row but not forming a distinct border, projecting strongly at upper ends. Costa rather stout, ending well below the leaf apex to nearly percurrent, lacking secondary pigments. Gemmae absent.
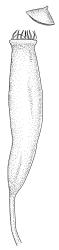
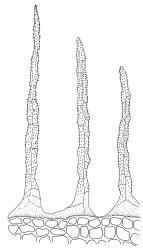
Paroicous or synoicous. Perichaetia basal, appearing lateral due to innovation, with leaves yellow, somewhat smaller, less toothed, and less strongly costate than vegetative leaves. Setae (10–)15–33 mm, pale brown, erect; capsules erect or weakly inclined, oblong-cylindric, weakly asymmetric and curved, 2.5–4.0 × c. 0.9 mm, with an ill-defined, tapered neck c. ⅓ the total capsule length, pale brown at maturity; annulus well-differentiated, persistent at mouth; operculum bluntly conic, sometimes weakly apiculate. Peristome single or double; exostome absent or highly reduced and scarcely extending beyond mouth; endostome segments linear, weakly nodose (as illustrated) to appendiculate (not illustrated), non-perforate, c. 225–300 µm long (a few sometimes anastomosing or short and irregular), arising from a short, pale, and smooth basal membrane usually extending 15–75 µm beyond mouth. Spores 18–25 µm, nearly smooth.
Scott & Stone 1976, pl. 53; Magill 1987, fig. 96, 1–11.
The suberect, slightly asymmetric, oblong-cylindric capsules, basal perichaetia, and the functionally single peristome arising from a low endostomal membrane collectively permit its easy recognition in the field. The blunt, rather widely spreading marginal teeth and thin-walled, elongate laminal cells provide further distinction, especially in the absence of capsules. However, this paroicous species commonly fruits. The development of the endostomal basal membrane and of appendiculae on the endostomal segments is quite variable.
Confusion is most likely between M. bryoides and Pohlia nutans. The lack of red pigments in the stem and costa, the thinner-walled laminal cells, and the basal perichaetia of M. bryoides help distinguish it from the Pohlia. The upper marginal teeth in M. bryoides are stronger and project from the leaf margin at a wider angle than those of Pohlia nutans. The two species could scarcely be confused when fruiting, given the marked differences in capsule stance and the nature of the peristomes.
NI: S Auckland, (Ātiamuri to Taupō and vicinity), Taranaki (Mt Egmont), Wellington (Mt Ruapehu); SI: Canterbury, Westland (Ōtira Gorge), Otago, Southland (Eyre Range).
Austral. Tasmania*, mainland Australia*, S Africa*. Shaw & Ramsay (2013) recorded it from Madagascar.
On soil in lowland to subalpine scrublands, grasslands, and in southern beech (mostly Fuscospora cliffortioides dominated) forest. Often on thin soil over dry greywacke, schist, or basalt outcrops. This is a characteristic and common species of sheltered loess banks and outcrops east of the Main Divide on the South I. It is a common species on Banks Peninsula and inland Otago L.D. Occurring to c. 1550 m (Mt Ruapehu) on the North I., and from c. 250 m (near Berwick, Otago L.D.) to c. 1500 m (Mt St Patrick, Canterbury L.D.) on the South I. Often associated with Bartramia mossmaniana, B. papillata, Ditrichum spp., and Philonotis scabrifolia.
Although only limited South African material of M. bryoides has been available for study, Magill’s (1987) description and illustrations suggest that the species may be more variable with respect to endostome form (with segments sometimes more appendiculate and with a greater tendency to anastomose) in South Africa than in N.Z. Most capsules in the African lectotype of Schizymenium bryoides are too old to permit detailed examination. An illustration of the peristome in Wilson’s herbarium shows a double peristome with short, rounded, and apparently smooth teeth, and irregular, strongly appendiculate endostome segments. There is also a tendency for South African material to have more strongly costate (percurrent to shortly excurrent) vegetative leaves than N.Z. material.
Scott & Stone (1976, p. 295) indicate that in some Australian populations a rudimentary exostome is sometimes present “but each tooth fades out above instead of continuing to a triangular point. The appearance is very much that of wax teeth melted by heat at the tips.” Weakly developed exostome teeth can be observed in some N.Z. collections, including B. Molesworth 115 (WELT M 009328) from near Queenstown, a collection cited by Sainsbury (1945).
The lectotype (BM 000870621) of Schizymenium bryoides Harv. was annotated in herb. BM by Shaw in April 1984. It is a very ample specimen and has associated notes and peristome, annulus, and leaf drawings by Wilson. Shaw’s lectotypification was never published, and Shaw & Ramsay (2013) merely cited a type from southern Africa. The citation by Magill (1987, p. 338) of a holotype in BM is also incorrect. The numerous replicates of the Harvey specimen in BM and other herbaria require a lectotype to be formally designated, as done here. Only a single isolectotype (BM 000870622!) has been examined non-electronically.
Three syntypes for Mielichhoferia ecklonii Hornsch., all from the Cape of Good Hope region, were designated by Hornschuch; two were collected by Ecklon and the third by Zeyher. Images of several duplicates of these collections, kept in various herbaria, can be viewed on JSTOR Global Plants (accessed 5 March 2018). A duplicate of the Ecklon collection from Löwenrücken was annotated in BM as a potential lectotype by Shaw in April 1984, but this lectotypification seems not to have been published. Magill annotated the same duplicate (BM 000870619), as did Shaw, and cited it in his 1987 treatment of southern African Mielichhoferia. A different duplicate of the Löwenrücken collection (BM!) has been seen during the preparation of this account and it is cited above as a syntype. Formal designation of a lectotype for this name is desirable, but not done here.
The lectotype of Mielichhoferia australis Hampe was selected independently by both J. Shaw and Fife in herb. BM (000983478!), but neither selection was published. There are isotypes in BM (BM 000983479 and BM 000983480) (images seen online, JSTOR Global Plants, accessed 5 March 2018), indicating the desirability of formal lectotypification, and this is done here for clarity. The statement by Shaw & Ramsay (2013) that the collector of the lectotype is unknown is perplexing as the specimen in question was clearly collected by Ferdinand Mueller. It is also perplexing that Shaw & Ramsay incorrectly cite a holotype in BM when three specimens are present and two of them bear the “Herb. Hampe” stamp.