- ≡ Dicranum obesifolium R.Br.bis, Trans. & Proc. New Zealand Inst. 29: 462 (1897)
- ≡ Braunfelsia obesifolia (R.Br.bis) Dixon, Bull. New Zealand Inst. 3: 79 (1923)
- = Eucamptodon petriei Broth., Öfvers. Finska Vetensk.-Soc. Förh. 40: 161 (1898)
- ≡ Braunfelsia petriei (Broth.) Broth., Nat. Pflanzenfam. [Engler & Prantl] 1(3), 321 (1901)
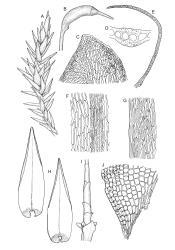
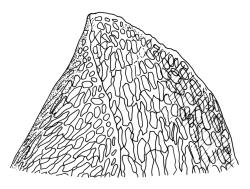
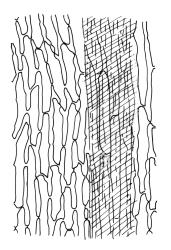
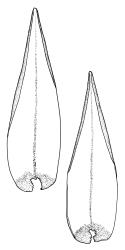
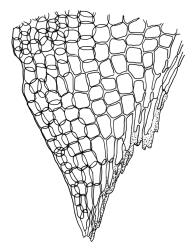
Plants robust, pale yellow-green and dull or rarely black when fresh, becoming gold-brown in older dried specimens, forming cushions or loose turves. Stems 30–120 mm, usually branched by forking, sometimes growing horizontally and functioning as stolons, in cross-section with 2–3 layers of cortical cells, rather firm-walled medullary cells, and a weak and ill-defined central strand, sparsely matted throughout by short, tangled, smooth, and pale brown or nearly white rhizoids, or sometimes rhizoids appearing absent. Shoots c. 12 mm wide (when leaves are wide-spreading) or narrower, 3–7 mm wide (when leaves are erect-appressed) when moist, variably cuspidate (sometimes strongly and sharply so). Leaves mostly widely spreading, occasionally closely erect-appressed, not secund, neither plicate nor rugose, little altered when dry, ovate, rounded or broadly acute at apex, clasping and ± auriculate at insertion, entire, (4.0–)6–8 × 1–5–3 mm (under cover slip), tubulose; mid laminal cells elongate and irregular, mostly (50–)90–120 × c. 12–15 µm and 5–8:1, thick-walled and highly porose, with highly irregular cells at extreme apex; juxtacostal cells at mid leaf not differentiated; border narrow but well defined, extending from alar cells nearly to apex, c. 3–4 cells and 12 µm wide at mid leaf; cells of leaf base scarcely differentiated except for 3–4 rows of pigmented cells at insertion; alar cells abruptly differentiated, auriculate, extending c. ⅔ or more to costal base and c. 8–10 cells up the margin, mostly oblong, subquadrate, or somewhat irregular, unistratose, weakly inflated, brown, yellow, or hyaline, thin- or firm-walled, not collenchymatous, the largest c. 50–60 × 35 µm, often with some cells at the upper limit of the alar group very strongly porose and with a primordial utricle). Costa narrow and weak (variable in a single shoot and sometimes appearing absent), occupying c. 1/40 to 1/15 the widest part of leaf, ending far below the apex or percurrent, smooth abaxially, in mid leaf cross-section completely lacking wings, 3–5 cell layers thick and c. 60–75 µm wide, with 4–6 guide cells in 1(–2) layers, and c. 8–12 abaxial and c. 5 adaxial stereids each in ± single layers.
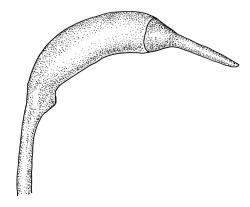
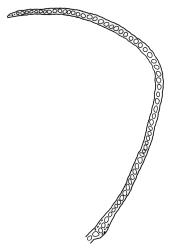
Pseudautoicous. Perichaetial leaves sheathing (c. ⅓ the seta length) and strongly concave, elliptic, broadly rounded or truncate at apex, with costa extremely weak and not forming an arista. Dwarf males embedded in rhizoids of sterile or ♀ plants. Setae single, c. 22–27 mm, straight, not twisted, red-brown; capsules curved, cylindric, 3.0–3.5 mm, strumose, smooth when moist, non-sulcate and constricted below the mouth when dry; exothecial cells firm-walled and elongate, variable in shape. Peristome teeth c. 640–700 × 120 µm, papillose-striolate in lower ½–⅔, baculate near apex. Spores 18–21 µm.
Apart from its problematic Tasmanian congener, D. obesifolium is most often confused with Pulchrinodus inflatus. The two species differ in numerous gametophytic features, of which the more readily observed are the non-rugose, singly costate, and apically rounded nature of the vegetative leaves in D. obesifolium. In P. inflatus the vegetative leaves are strongly rugose, ecostate or inconspicuously doubly costate, and have a small, reflexed acumen. Also, D. obesifolium is a high-elevation plant which frequently fruits, while P. inflatus occurs at lower elevations and has not been found bearing sporophytes.
NI: Hawke’s Bay or Wellington (Ruahine Ranges); SI: Nelson (Little Wanganui River, Cobb Valley, Mt Arthur Range, Lake Peel, Mt Mantell), Canterbury (Lewis Pass, Arthur’s Pass, Godley River, Little Mt Peel), Westland (Northern Olivine Range, Ōtira Valley, Kelly Range, Rahu Saddle), Otago (Mt Shrimpton), Southland (Nancy Sound, Gertrude Valley, Cozette Burn, Mt Burns, Takahē Valley, Tākitimu Range, Eyre Mountains).
Probably endemic.
Apart from a single poorly documented collection from the Ruahine Ranges by A.P. Druce (WELT M014859), this species is restricted to areas west of or close to the Main Divide on the South I. Occurring most commonly in alpine Chionochloa grassland (often C. rubra or C. pallens dominated) or shrubland, and often in sheltered, damp depressions beneath tussocks or shrubs or at the base of rock outcrops. Also occurring at the margins of boggy tarns or lakes, and there often in association with Schoenus pauciflorus, and occasionally submerged. On the Mt Arthur Range, D. obesifolium occurs on the sides of limestone dolines and it also occurs in Lophozonia menziesii dominated southern beech forest developed over marble bedrock. Dicranoloma obesifolium is (with D. robustum) one of only two species in the genus that commonly occurs above the tree line. Breutelia elongata, D. robustum, Racomitrium crispulum s.l., R. pruinosum, Rhacocarpus purpurascens and Sphagnum are often associated. Ranging from 885 m (Little Wanganui River) to 1650 m (Temple Basin in Arthur’s Pass) elevation.
The presumed holotype (BM) consists of a single, branched, and sterile stem.
The spreading and apically rounded leaves together with the dull yellow-green fresh coloration of representative material of this species make it unlikely to be confused with any of its congeners. There is a tendency in D. obesifolium for stems to grow horizontally (as stolons) and give rise to erect secondary stems. Dicranoloma obesifolium is the only N.Z. species of the genus in which the dry mature capsules are not plicate. The struma at the capsule base is more pronounced here than in any of its N.Z. congeners. A small number of short, broadly ovate and strongly spreading leaves are normally present at the base of the perichaetium.
However, D. obesifolium is a variable species, and one Southland collection (J.K. Bartlett 23190; CHR 447752 & WELT M007491from Percy Saddle) has been referred by both Bartlett & Frahm (1983) and Klazenga (2003) to the Tasmanian D. eucamptodontoides. This collection, in addition to D. Glenny 5133 (CHR 454197 & WELT M029309) from Symmetry Peaks in the Eyre Mountains (Southland L.D.) and A.J. Fife 10057 (CHR 532469) from the Kelly Range (Westland L.D), is problematic. All these collections compare well to Tasmanian material of D. eucamptodontoides housed at WELT. Type material of D. eucamptodontoides (Broth. & Geh.) Paris [Index Bryol. ed., 2, 2: 26 (1904)] has also been seen in NY (267975!).
One feature separating the two species, according to Klazenga (2003), is the relative length of the costae. Klazenga considered that the costae end far below the leaf apex in D. obesifolium but are percurrent in D. eucamptodontoides. However, numerous N.Z. collections of D. obesifolium have percurrent costae in some or most of their leaves (e.g., CHR 428940, J. Child 4383 from Gertude Saddle). Klazenga also considered the alar cells of D. eucamptodontoides to be uniquely collenchymatous. However, the interpretation of the alar cells here is problematic, and my interpretation of both J.K. Bartlett 23190 and D. Glenny 5133 is that the cells at the upper limit of the alar group are very strongly porose and some have their contents strongly pulled away from the cell walls when dry (thus exhibiting a "primordial utricle"). I would not interpret the N.Z. material to have collenchymatous alar cells; in this regard, this material matches the two Tasmanian collections studied.
The black coloration and the distinctly cuspidate shoot apices of the Southland and the Kelly Range material are striking features which contrast with the distinctive pale yellow-green and dull (when fresh) and gold-brown (when dry) and weakly or non-cuspidate shoots of more representative D. obesifolium. In both these features the Southland and the Kelly Range material agree with Tasmanian D. eucamptodontoides. The marginal border of the Southland and the Kelly Range material is much less conspicuous above mid leaf, and the alar groups appear to be less auriculate than in most D. obesifolium.
On balance it seems better to apply the name D. obesifolium (R.Br.bis) Broth. to these aberrant collections and to exclude D. eucamptodontoides from the N.Z. flora. If D. obesifolium and D. eucamptodontoides are eventually proven to be conspecific, the Tasmanian name would have nomenclatural priority.