- ≡ Hypnum cuspidatum Hedw., Sp. Musc. Frond. 254 (1801)
- ≡ Acrocladium cuspidatum (Hedw.) Lindb., Musci Scand. 39 (1879)
- ≡ Calliergon cuspidatum (Hedw.) Kindb., Canad. Rec. Sci. 6: 72 (1894)
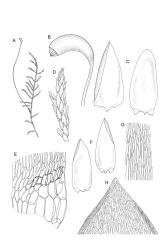
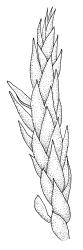
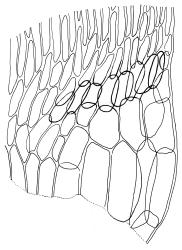
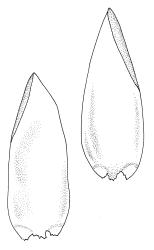
Plants medium-size to robust, yellow- or brown-green. Stems subpinnately or irregularly branched, commonly to c. 100 mm, in cross-section with a well-developed hyaloderm surrounding several layers of thick-walled cells and with a central strand; rhizoids not seen; stem and branch tips cuspidate by erect and overlapping leaves. Stem leaves erect and imbricate near stem tip, erect-spreading to spreading on lower portion of stem, broadly ovate, broadly obtuse or rounded at apex, with or without a short apiculus, slightly narrowed to insertion, not plicate, concave, entire, not bordered, inrolled at margins near apex, c. 2.0–2.5 × 0.9–1.0 mm (under cover slip); mid laminal cells linear and flexuose, mostly c. 75–100 × 5–6 µm, firm-walled, not porose, becoming shorter near apex; basal cells shorter, incrassate, and porose in several rows; alar group large and strongly defined, ± excavate, extending about ¼ the leaf width, the cells strongly inflated, thin-walled and hyaline. Costa absent or very weak and double. Branch leaves tightly or loosely imbricate near branch tip, erect-spreading to spreading below, narrower and more lanceolate than stem leaves, ± acute at apex, strongly concave and becoming subtubulose above. Axillary hairs numerous and easily observed. Pseudoparaphyllia foliose, robust, rounded at apices. Paraphyllia absent.
Dioicous. Perichaetial leaves erect, plicate, acuminate. Perigonia not seen. Setae elongate (c. 50–75 mm in N.Z. material), smooth, red; capsules cylindric or obovoid, strongly curved and ± horizontal, wrinkled and strongly constricted below the mouth when dry, c. 3.0 mm; stomata numerous in neck; annulus well-differentiated; operculum conic. Peristome hypnoid; exostome teeth pale yellow-brown, transverse-striolate below, shouldered and bordered; endostome well-developed, with a high basal membrane, and paired, appendiculate cilia. Calyptra cucullate, naked. Spores finely papillose, 18–21 µm in N.Z. material.
Crum & Anderson 1981, fig. 495; Smith 2004, fig. 298, 8–12; Meagher & Fuhrer 2003, p. 55; Sainsbury 1955, pl. 71, fig. 2 (as Acrocladium cuspidatum).
Although it is a distinctive plant readily recognised by its branching pattern, cuspidate stem and branch tips, and effectively ecostate stem and branch leaves with strongly inflated alar cells, Calliergonella cuspidata is sometimes confused with Acrocladium chlamydophyllum. The two species differ in many ways. Calliergonella cuspidata grows in moister habitats and is a more erect, less julaceous, and more pinnately branched plant than A. chlamydophyllum. The cuspidate stem tips of C. cuspidata contrast with the julaceous but non-cuspidate stem tips of the Acrocladium. The stem leaf mid laminal cells of Calliergonella are non-porose in contrast with the strongly porose mid laminal cells of A. chlamydophyllum.
Calliergonella is also occasionally confused with Pseudoscleropodium purum. The non-reflexed leaf apex and the poorly developed costae in C. cuspidata are sufficient to distinguish it from P. purum. The latter is also a more lustrous and more robust plant growing in drier situations.
K; NI: N Auckland, S Auckland (Rangiriri, Hardcastle Lagoon), Gisborne (Raukokore River), Taranaki, Wellington; SI: Nelson, Canterbury, Westland, Otago, Southland; St; A.
Adventive. Tasmania*, Australia*; widespread in the northern hemisphere, and recorded from several localities in South America (see Hedenäs 2003) and elsewhere (cf. Buck 1998, p. 224).
Occurring in a wide range of mostly disturbed and damp sites including pastures, road margins, and ditches; often forming mats of several square metres. Also occurring in damp openings in indigenous forest and subalpine scrub and submerged in slowly moving or still water. It is particularly well-developed in seepages in calcareous areas. On North I. from near sea level to at least 1250 m (Silica Rapids, Wellington L.D.); on South I. from near sea level to at least 740 m (Broken River, Canterbury L.D.) and 1370 m (Garvie Range, Otago L.D.), but with the bulk of records from low elevations. Herbarium collections suggest that this species is particularly abundant in eastern Otago. Frequently associated mosses include Breutelia pendula, Hypnum cupressiforme, and Pseudoscleropodium purum.
While most frequent in damp situations rather than aquatic situations, Calliergonella cuspidata also grows completely submerged, both in habitats with varying and constant water levels. When submerged the growth form is more lax, the branching more irregular, and leaves somewhat longer (stem leaves to c. 3.0 mm) than in terrestrial forms. There are several records of it (apparently from depths of up to 2 m) in the very clear waters of the limestone Te Waikoropupū ("Pupu") Springs in Nelson L.D.
Calliergonella cuspidata is probably an early European-era introduction to the N.Z. flora. There are no citations for this species in either Flora Novae-Zelandiae (Wilson 1854) or the Handbook of the New Zealand Flora (Hooker 1867). The oldest confirmed N.Z. collections were made by W. Bell at Pelichet Bay (Dunedin) in 1888 and 1890 and reported as "another northern moss now first recorded in New Zealand" by Beckett (1897). Pelichet Bay subsequently became known as Lake Logan and was eventually "reclaimed"; it is presently known as Logan Park.
Karczmarz (1971) recognised several infra-specific taxa within what he termed Calliergon cuspidatum and named one Nelson L.D. specimen (presumably from Te Waikoropupū Springs) as his var. fluitans. His infra-specific taxa have received little acceptance in the literature and are not recognised here. Like Crum & Anderson (1981), I believe that most of Karczmarz’s infra-specific entities are better considered as mere ecological forms.
Axillary hairs can be easily observed in Calliergonella by carefully removing leaves from terminal shoots. Staining with a proprietary dye such as toluidine blue can facilitate observation but is usually not required. In form the hairs agree with the illustration of Hedenäs (1990, fig. 106) and consist of one basal cell and c. 3–5 uniform and cylindric upper cells. In my limited experience the upper 3–5 cylindric cells are yellow-brown in colour and the basal cell is the least pigmented. This pattern contrasts with Hedenäs’s illustration (see also the description of Hedenäs 2003, p. 80). Representative axillary hairs in Calliergonella are c. 100–180 µm long; the yellow-brown cylindric cells absorb toluidine blue more strongly than the basal cell. However, in Calliergonella, as, indeed, in the entire family, axillary hairs seem to be of little taxonomic value.
Sporophytes are very rare in N.Z. The number of endostomal cilia was reported by Hedenäs (2003) as 2–4 for the genus and by Crum & Anderson (1981) as 3–4; only paired cilia have been seen in N.Z. material.