- ≡ Pohlia clavata Schimp., Ann. Sci. Nat., Bot. sér. 2, 6: 148 (1836)
- ≡ Gemmabryum clavatum (Schimp.) J.R.Spence & H.P.Ramsay, Phytologia 87: 66 (2005)
- ≡ Imbribryum clavatum (Schimp.) J.R.Spence & H.P.Ramsay, Telopea 15: 146 (2013)
- = Bryum clavatum var. extenuatum Hook.f. & Wilson in Wilson, Bot. Antarct. Voy. II (Fl. Nov.-Zel.) Part II, 84 (1854)
- ≡ Bryum curvicollum var. extenuatum (Hook.f. & Wilson) Hook.f., Handb. New Zealand Fl. 442 (1867)
- = Bryum erythrocarpoides Müll.Hal. & Hampe, Linnaea 26: 495 (1855)
- = Bryum curvicollum Mitt. in Hooker, Handb. New Zealand Fl. 442 (1867)
- = Bryum varians Müll.Hal., Bot. Jahrb. Syst. 5: 87 (1884)
- = Bryum laevigatulum Broth., Öfvers. Finska Vetensk.-Soc. Förh. 40: 176 (1898)
- = Bryum levieri Müll.Hal., Hedwigia 37: 92 (1898)
- = Bryum macroerythrocarpum Müll.Hal., Hedwigia 37: 92 (1898) – as macro-erythrocarpum
- = Bryum gracilithecium R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 453 (1899)
- = Bryum hapukaense R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 455 (1899)
- = Bryum heterofolium R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 458 (1899)
- = Bryum kirkii R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 454 (1899) nom. illeg., non Bryum kirkii Broth. 1898
- = Bryum linearifolium R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 453 (1899)
- = Bryum macrocarpum R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 455 (1899) nom. illeg., non Bryum macrocarpon Hedw. 1801
- = Bryum ventricosum R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 454 (1899)
- = Bryum webbianum R.Br.bis, Trans. & Proc. New Zealand Inst. 31: 452 (1899)
- = Bryum schauinslandii Müll.Hal., Abh. Naturwiss. Vereins Bremen 16: 502 (1900)
- = Bryum foresteri R.Br.bis, Trans. & Proc. New Zealand Inst. 35: 334 (1903) – as foresterii
- = Bryum luteolimbatum Broth., Proc. Linn. Soc. New South Wales 41: 589 (1916)
Non Bryum clavatum Hook.f. & Wilson, hom. illeg. (1854)
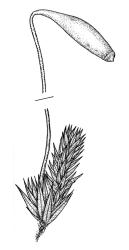
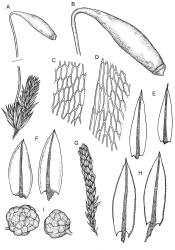
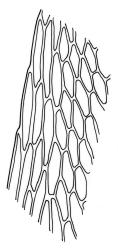
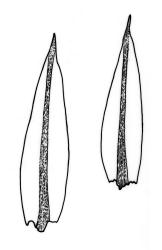
Plants brown- or red-green or bright green, small to medium-sized, not or slightly lustrous. Stems red-brown, to c. 5–10 mm, much branched below (often by subperichaetial innovation), beset with brown, papillose rhizoids below, in cross-section with firm-walled cortical cells and an ill-defined central strand. Leaves evenly spaced on stem, erect when moist and little altered when dry, oblong-lanceolate, acute and mostly stoutly cuspidate at apex, variable in size (mostly 1.0–1.8(–2.0) mm in fertile stems; mostly 0.7–1.2 mm on innovative branches and in sterile material) and with lamina mostly ≥0.85 the total leaf length, weakly concave, not plicate, variably coloured, entire or less often weakly denticulate, bordered, narrowly recurved on one or both sides or occasionally plane, not decurrent; upper laminal cells oblong hexagonal, firm-walled, c. 27–45 × 9–12 µm and 3–4:1, ± shorter near apex, more oblong and slightly longer (to c. 54 µm) below; marginal cells linear and thick-walled, forming a border of variable width (mostly (1–)3–6 cells wide at mid leaf), extending to near leaf base, becoming obscure near apex; basal cells subquadrate in a few rows, not pigmented. Costa stout, yellow- or red-brown, or occasionally golden-pink, excurrent to form a stout, acute, and entire cusp, less often percurrent. Bulbils sometimes present in upper leaf axils, broadly ellipsoid, c. 250 × 100 µm, red-brown at base and with 4–5 apical primordial leaves. Tubers sometimes present, red-brown, irregular, mostly 75–150 µm in greater diam., with protuberant cells.
Dioicous. Perichaetia in branch axils, usually near base of plant; perichaetial leaves triangular-lanceolate. Perigonia budlike in the axils of lower branches, c. 0.5 mm, the inner bracts smaller and more concave than vegetative leaves, with filiform, 5–6-celled paraphyses. Setae (7–)15–22(–40)mm, red-brown; capsules inclined, horizontal or cernuous, dark purple, narrowly clavate, with a well-defined neck (not well-illustrated here) c. ½ the total length, strongly curved, and wrinkled when dry, (2.5–)3.5–5(–7.0) mm long; operculum conic, apiculate. Exostome pale; endostome not adhering to teeth, with broad, perforate segments c. the height of exostome teeth, cilia rudimentary, lacking, or well developed and nodose or rarely appendiculate (highly variable even in single capsule) 1–3(–4) in number. Spores (12–)20–27 µm.
Dixon 1926, pl. 9, p.p. (as B. curvicollum); Ochi 1970, figs 13–16 (as B. erythrocarpoides); Spence & Ramsay 2006, pl. 40, A–G (as Gemmabryum clavatum). The illustration in Seppelt 2004 (fig. 37, as B. clavatum) may be from anomalous material, with some costae shown as ending well below the leaf apices.
Species of the "B. erythrocarpum complex" have shorter, more pendent capsules with broader endostomal segments and better developed, more appendiculate cilia than the usual expressions of B. clavatum. Sterile material of some members of the "B. erythrocarpum complex" (e.g., B. duriusculum, B. sauteri) can also be difficult to distinguish from B. clavatum, but the denticulate vs the normally entire (in B. clavatum) leaf awn usually allows distinction. The shape and diameter of the frequently produced red-brown, ± isodiametric tubers of B. clavatum clearly distinguish it from those two species.
Confusion between B. clavatum and other Bryum species can also occur. Bryum caespiticium (which can produce tubers) has more finely excurrent costae, consistently appendiculate cilia, broadly perforate endostomal segments, and smaller spores than B. clavatum. Sterile material of B. dichotomum can be difficult to differentiate from B. clavatum and the most reliable differences are the lack of a leaf border in the former, and the differing leaf shape.
The confusing variability of B. clavatum and the lack of clearly delimiting morphological boundaries between it and other species suggest that further study, perhaps using molecular techniques, would be worthwhile, but such investigations are beyond the scope of this Flora.
K; NI: N Auckland, S Auckland (Waingaro), Gisborne, Hawke's Bay, Taranaki, Wellington; SI: Nelson, Marlborough, Canterbury, Westland (Barrack Creek, Franz Josef), Otago; Ch; A. Reported from M by Seppelt (2004) and Spence & Ramsay (2006).
Austral. Tasmania*, mainland Australia*, southern South America. Reported from South Africa by Ochi (1970, as B. erythrocarpoides). Spence & Ramsay (2006) treated B. clavatum in Gemmabryum, and recorded a wider distribution that included New Guinea, Lord Howe I., and the South Pacific.
In seepages on outcrops of a wide variety of rock types (including basalt, greywacke, papa, limestone, and ultramafics) and on soil; often in wet road cuttings. Frequently coastal, but also common away from coastal influence. Forming dense cushions to at least 150 mm diameter. On North I. from sea level to c. 1220 m and to at least 850 m on South I. Dicranella cardotii is a frequent associate.
The commonly produced, narrowly clavate and strongly curved capsules (poorly illustrated here), in association with the stoutly excurrent costa, firm-walled laminal cells, entire and bordered leaves, and its usual occurrence on irrigated or damp ledges facilitate the recognition of B. clavatum. In its most common expression, with curved, inclined, dark purple capsules exceeding 4 mm in length, and relatively short setae, there is no species in N.Z. likely to be confused with B. clavatum. However, B. clavatum is an extraordinarily variable species and worthy of more detailed study. Microscopically, the relatively large spores are a useful recognition feature. Capsule dimensions and degree of endostomal cilia development vary considerably, even in single capsules. Endostome cilia range from nearly absent to 2–3, moderately developed, and nodose (the most frequent pattern), to rarely 3–4 and appendiculate.
Coastal material of B. clavatum, when sterile, tends to form distinctive compact cushions, usually on irrigated outcrops. A distinct appearance is provided by much-branched and closely packed stems with firm, dark green leaves scarcely exceeding 1 mm in length and stoutly excurrent, smooth costae. In such material the leaf border can be obscure (only 1–2 cells wide) and upper laminal cells somewhat smaller (c. 20–24 µm long) than in the description.
Ochi's (1970) extensive illustrations (as B. erythrocarpoides) convey an impression of the variability of this species. In his illustrations, the costae are invariably percurrent to excurrent; these illustrations accord with my own observations.
However, according to Beever et al. (1992) the costae of this species are "quite variable in length, and may fail before the apex or be excurrent in a stout point". According to J. Beever (pers. comm. 8 Apr. 2015) the late John Linzey also believed that leaves of this species, particularly those of innovative branches, could have costae failing in or below the leaf apices. Such leaves are illustrated from Macquarie I. by Seppelt (2004, fig. 37).
Confusion remains concerning the best name for what is here termed B. clavatum (Schimp.) Müll.Hal. The confusion is due mostly to questions surrounding the typification of the Chilean basionym Pohlia clavata Schimp. Schimper’s protologue is clear that the type of P. clavata Schimp. was collected by Bertero in Quillota province in Chile in 1829. Type material is present in PC (PC 136536) and in BM (BM 517977); both can be viewed online (at JSTOR Global Plants).
Ochi annotated, in 1973, the fragmentary type in BM (517977); as: "Isotype of Bryum clavatum (Schimp.) C.Muell. Bryum erythrocarpoides C.Muell. & Hamp. seems to be conspecific with this species". The limited detail of this specimen observable online is consistent with Ochi’s suggestion that the two taxa are conspecific.
The Schimper name was also applied to Australian material by Spence & Ramsay (2006, p. 297, as Gemmabryum clavatum (Schimp.) J.R.Spence & H.P.Ramsay), although they incorrectly based their concept of Pohlia clavata Schimp. on an early N.Z. collection by Logan, rather than the Bertero collection. Despite these irregularities, Spence and Ramsay’s application of this name to Australasian material, albeit neither of their proposed nomenclatural combinations for it, is adopted here.
Of the four syntypes of B. curvicollum Mitt. (the name applied to B. clavatum by Sainsbury 1955), only the ample material collected by Logan in BM has been seen. A duplicate of the Logan collection in herb. Mitten would be the most appropriate lectotype for this name.
Material to which J.T. Linzey (unpublished) and some subsequent workers gave the unpublished name "Bryum ACC" is confusing but is here treated as B. clavatum. One of the features of so-called "B. ACC" is its irregular red-brown tubers of 60–105 µm diam., with individual cells moderately protruding. These are indistinguishable from tubers in some populations of B. clavatum and some non-N.Z. species (especially B. klinggraeffii Schimp.) of the "B. erythrocarpum complex". Material of "B. ACC" can differ from the usual forms of B. clavatum by several loosely correlated features, including: a denticulate leaf awn, more compact basal leaf cells, pendent, shorter, and more symmetric capsules, longer and appendiculate endostomal cilia (often in groups of 3), more widely perforate segments, and smaller spores (c. 12–16 µm diam.). All these features, as well as tuber production, are associated with the "B. erythrocarpum complex". This, indeed, is the relationship that Linzey (unpublished) proposed for "B. ACC". However, the correlation between these character states is not strong. The confusing variety of character state combinations is not unlike what might occur in a hybrid swarm, although it does not result in malformed spores. The overall range of variation suggests that "B. ACC" should be included within B. clavatum.