- ≡ Physcomitrella bartlettii Fife, Lindbergia 8: 103 (1982)
Physcomitridium readeri sensu Sainsbury (1955)
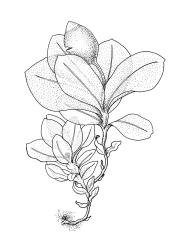
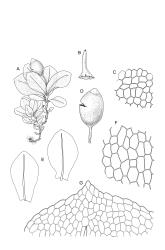
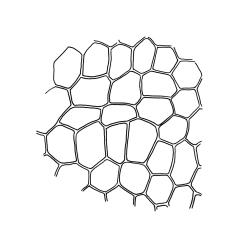
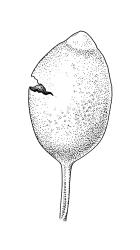
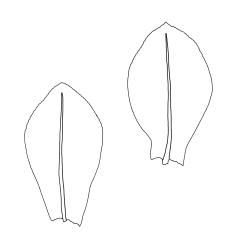
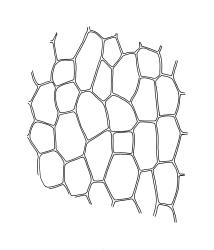
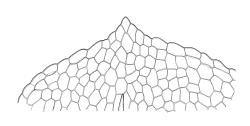
Plants small, gregarious, yellow-green. Stems commonly c. 2–4 mm, unbranched, branched once by subperigonial innovation or rarely branching repeatedly by innovations, red-brown, in cross-section with a small central strand, a parenchymatous medulla, and 1–2 cortical layers of firm-walled cells, beset below with smooth, red-brown rhizoids. Leaves obovate to oblong-obovate, obtuse or broadly rounded at apex, 1.5–2.8 × 0.6–1.6 mm, erect-spreading, weakly concave, contorted when dry, plane at margins, entire or weakly and bluntly toothed above by projecting cells ends; upper laminal cells oblong-hexagonal (some ± irregular), thin-walled, (25–)45–60(–80) × c. 20–36 µm, becoming larger and more oblong near leaf base, and slightly smaller near apex; marginal cells not or weakly differentiated; alar cells scarcely differentiated. Costa yellow-brown, c. 40 µm wide near base, ending 3–10 cells below leaf apex, in cross-section with a small central stereid group and large abaxial and adaxial cells. Axillary hairs as per family.
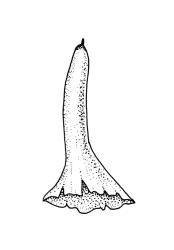
Autoicous or perigonia not observable. Perigonia commonly terminal and overtopped by a perichaetial innovation. Setae red-brown, straight, weakly dextrorse, 0.8–1.5 mm (excluding the vaginula, which is c. 0.3 mm long); capsules erect, weakly exserted, ellipsoid, 0.8–1.5(–1.8) mm, broadly obtuse, with a distinct and often somewhat inflated neck (⅕–)¼–⅓ the total length of the capsule (not well illustrated here), splitting irregularly at maturity and/or rarely splitting transversely ± at equator; columella not seen (presumably resorbed); exothecial cells oblong or ± irregular, thin-walled, not thickened at corners, mostly c. 45–55 in greater diam., moderately differentiated and thicker-walled at capsule apex; rarely forming a ± functional operculum; peristome nil; stomata numerous (c. >40) and weakly immersed; operculum absent or sometimes poorly differentiated and not functional. Calyptra mitrate or becoming split on one side, c. 1 mm long and covering only the capsule apex, deciduous or persistent. Spores spherical or subreniform, 28–38 µm, red-brown, uniformly spinose, lacking a trilete scar and not persisting in tetrads.
Fife 1982b, figs 11–21 (as Physcomitrella bartlettii).
Features that distinguish B. bartlettii from the rarer Physcomitrium pusillum and Physcomitridium readeri are discussed under those species.
NI: N Auckland (Whangārei), S Auckland, Hawke’s Bay (Wairoa), Wellington (Somes I., Taihape, Carterton, Paekākāriki Hill Road, Petone); SI: Nelson (Wakefield, Westport), Canterbury (Christchurch vicinity, Banks Peninsula), Otago (Blueskin Bay, Evansdale, Kelso), Southland (Riverton). Ch (Pitt I.)
Endemic.
On damp, often recently disturbed silt or clay, often at the margins of streams or drainage ditches. Sometimes on recently deposited alluvium and tolerant of some coastal salt spray. Associated taxa include Eurhynchium praelongum, Funaria hygrometrica, Hennediella macrophylla, Physcomitridium readeri, Physcomitrium pusillum, P. pyriforme, Pseudephemerum nitidum, Tortula truncata, and Weissia controversa. Collections have been made throughout the year, but capsules are most frequently mature in early summer. Known only from low elevation (<100 m) sites, with the exception of the Kelso collection, probably made at c. 200 m elevation.
Sexuality, although sometimes clearly autoicous (and with the stem branching by subperigonial innovation), is often unclear and in many collections antheridia cannot be demonstrated.
Mature capsules of representative B. bartlettii usually have a weakly defined area of thicker-walled cells at the capsule apex (suggestive of an incipient operculum and rarely ± functional), but spore escape is most often by irregular fragmentation of the capsule wall. The spore sac readily separates from the exothecial cells in dissected capsules. The degree of differentiation of the exothecial cells near the capsule apex varies greatly between populations and even between plants in a population. In some, but probably not all, instances the differentiation of a small and ± functional operculum may be the result of hybridisation with Physcomitrium pyriforme (as in the Kelso material discussed below).
The absence of material of the relatively widespread B. bartlettii in the Sainsbury herbarium (at WELT) is perplexing.
Sainsbury’s (1955, p. 248) description of Physcomitridium readeri sensu Sainsbury is partly based on Bryobeckettia bartlettii collections made by E.A. Hodgson at Wairoa, Hawke’s Bay L.D. These are currently not present in the WELT-Sainsbury herbarium, but are represented by four duplicates in CHR, including CHR 548148. No identifiable Physcomitrium is present in the Hodgson collection.
Sainsbury also used collections by W. Martin, including Martin 807 of 29 Dec. 1948 from Kelso (Otago L.D.) when drafting his Physcomitridium readeri description. A part of this collection is preserved as CHR 266335 and it is a mixture of B. bartlettii, Physcomitrium pyriforme, and plants with inter-generic hybrid sporophytes. The hybrid capsules are mostly B. bartlettii ♀ × P. pyriforme ♂, borne on Bryobeckettia gametophytes. The resultant capsules are exserted, ellipsoid, with a well defined neck and an umbonate operculum, which in at least one plant was functional. In other capsules the umbonate operculum is not fallen. The spores in some of these capsules are highly malformed, with a considerable fraction persistent as tetrads. In one immature capsule with an apparently functional but attached operculum, the spores are well formed, uniformly spinose, and neither in tetrads nor trilete scarred. One example of an apparent P. pyriforme ♀ × B. bartlettii ♂ is also present; the spores here are variable in diameter (c. 18–33 μm) and the larger spores well developed, while some of the smaller spores are poorly formed. This Martin collection from Kelso material provides the most convincing N.Z. example of funariaceous inter-generic hybrids. No hybrid sporophytes have been seen in a scant duplicate (CHR 548151) of a Martin collection from the same locality gathered three days later (on 1 Jan 1949). No other collections of B. bartlettii have been seen with hybrid capsules.
Inter-generic hybrid capsules involving other genera in the Funariaceae (including the North American genus Aphanorhegma) have been documented (e.g., by Fife 1982a) and are well documented in culture experiments since the classic studies of von Wettstein (1932).